+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10415 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | SAGA DUB module bound to a ubiqitinated nucleosome | |||||||||
 Map data Map data | postprocess masked map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Coactivator / Transcription / Histone acetyltransferase / Histone deubiquitinase / GENE REGULATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationDUBm complex / RITS complex assembly / regulation of nucleocytoplasmic transport / transcription export complex 2 / nuclear mRNA surveillance / SLIK (SAGA-like) complex / post-transcriptional tethering of RNA polymerase II gene DNA at nuclear periphery / regulation of protein localization to chromatin / SAGA complex / poly(A)+ mRNA export from nucleus ...DUBm complex / RITS complex assembly / regulation of nucleocytoplasmic transport / transcription export complex 2 / nuclear mRNA surveillance / SLIK (SAGA-like) complex / post-transcriptional tethering of RNA polymerase II gene DNA at nuclear periphery / regulation of protein localization to chromatin / SAGA complex / poly(A)+ mRNA export from nucleus / positive regulation of RNA polymerase II transcription preinitiation complex assembly / protein deubiquitination / Ub-specific processing proteases / nuclear pore / mRNA export from nucleus / Maturation of protein E / Maturation of protein E / ER Quality Control Compartment (ERQC) / Myoclonic epilepsy of Lafora / FLT3 signaling by CBL mutants / Constitutive Signaling by NOTCH1 HD Domain Mutants / IRAK2 mediated activation of TAK1 complex / Prevention of phagosomal-lysosomal fusion / Alpha-protein kinase 1 signaling pathway / Glycogen synthesis / IRAK1 recruits IKK complex / IRAK1 recruits IKK complex upon TLR7/8 or 9 stimulation / Endosomal Sorting Complex Required For Transport (ESCRT) / Membrane binding and targetting of GAG proteins / Negative regulation of FLT3 / Regulation of TBK1, IKKε (IKBKE)-mediated activation of IRF3, IRF7 / PTK6 Regulates RTKs and Their Effectors AKT1 and DOK1 / Regulation of TBK1, IKKε-mediated activation of IRF3, IRF7 upon TLR3 ligation / IRAK2 mediated activation of TAK1 complex upon TLR7/8 or 9 stimulation / NOTCH2 Activation and Transmission of Signal to the Nucleus / TICAM1,TRAF6-dependent induction of TAK1 complex / TICAM1-dependent activation of IRF3/IRF7 / APC/C:Cdc20 mediated degradation of Cyclin B / Regulation of FZD by ubiquitination / Downregulation of ERBB4 signaling / APC-Cdc20 mediated degradation of Nek2A / p75NTR recruits signalling complexes / InlA-mediated entry of Listeria monocytogenes into host cells / TRAF6 mediated IRF7 activation in TLR7/8 or 9 signaling / RNA splicing / Regulation of pyruvate metabolism / NF-kB is activated and signals survival / TRAF6-mediated induction of TAK1 complex within TLR4 complex / Downregulation of ERBB2:ERBB3 signaling / Regulation of innate immune responses to cytosolic DNA / Pexophagy / NRIF signals cell death from the nucleus / Activated NOTCH1 Transmits Signal to the Nucleus / Regulation of PTEN localization / VLDLR internalisation and degradation / Synthesis of active ubiquitin: roles of E1 and E2 enzymes / Translesion synthesis by REV1 / TICAM1, RIP1-mediated IKK complex recruitment / Regulation of BACH1 activity / Translesion synthesis by POLK / MAP3K8 (TPL2)-dependent MAPK1/3 activation / Degradation of CDH1 / InlB-mediated entry of Listeria monocytogenes into host cell / JNK (c-Jun kinases) phosphorylation and activation mediated by activated human TAK1 / Activation of IRF3, IRF7 mediated by TBK1, IKKε (IKBKE) / Josephin domain DUBs / Downregulation of TGF-beta receptor signaling / Translesion synthesis by POLI / Gap-filling DNA repair synthesis and ligation in GG-NER / IKK complex recruitment mediated by RIP1 / Degradation of CRY and PER proteins / Regulation of activated PAK-2p34 by proteasome mediated degradation / PINK1-PRKN Mediated Mitophagy / TGF-beta receptor signaling in EMT (epithelial to mesenchymal transition) / TNFR1-induced NF-kappa-B signaling pathway / Autodegradation of Cdh1 by Cdh1:APC/C / TCF dependent signaling in response to WNT / Regulation of NF-kappa B signaling / APC/C:Cdc20 mediated degradation of Securin / N-glycan trimming in the ER and Calnexin/Calreticulin cycle / activated TAK1 mediates p38 MAPK activation / Asymmetric localization of PCP proteins / Ubiquitin-dependent degradation of Cyclin D / SCF-beta-TrCP mediated degradation of Emi1 / NIK-->noncanonical NF-kB signaling / Regulation of signaling by CBL / TNFR2 non-canonical NF-kB pathway / AUF1 (hnRNP D0) binds and destabilizes mRNA / transcription elongation by RNA polymerase II / NOTCH3 Activation and Transmission of Signal to the Nucleus / Negative regulators of DDX58/IFIH1 signaling / Negative regulation of FGFR3 signaling / Assembly of the pre-replicative complex / Fanconi Anemia Pathway / Peroxisomal protein import / Deactivation of the beta-catenin transactivating complex / Vpu mediated degradation of CD4 / P-body / Stabilization of p53 / Degradation of DVL Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
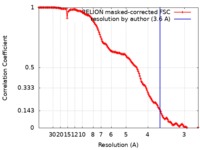
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Wang H / Cramer P | |||||||||
| Funding support |  Germany, 2 items Germany, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2020 Journal: Nature / Year: 2020Title: Structure of the transcription coactivator SAGA. Authors: Haibo Wang / Christian Dienemann / Alexandra Stützer / Henning Urlaub / Alan C M Cheung / Patrick Cramer /   Abstract: Gene transcription by RNA polymerase II is regulated by activator proteins that recruit the coactivator complexes SAGA (Spt-Ada-Gcn5-acetyltransferase) and transcription factor IID (TFIID). SAGA is ...Gene transcription by RNA polymerase II is regulated by activator proteins that recruit the coactivator complexes SAGA (Spt-Ada-Gcn5-acetyltransferase) and transcription factor IID (TFIID). SAGA is required for all regulated transcription and is conserved among eukaryotes. SAGA contains four modules: the activator-binding Tra1 module, the core module, the histone acetyltransferase (HAT) module and the histone deubiquitination (DUB) module. Previous studies provided partial structures, but the structure of the central core module is unknown. Here we present the cryo-electron microscopy structure of SAGA from the yeast Saccharomyces cerevisiae and resolve the core module at 3.3 Å resolution. The core module consists of subunits Taf5, Sgf73 and Spt20, and a histone octamer-like fold. The octamer-like fold comprises the heterodimers Taf6-Taf9, Taf10-Spt7 and Taf12-Ada1, and two histone-fold domains in Spt3. Spt3 and the adjacent subunit Spt8 interact with the TATA box-binding protein (TBP). The octamer-like fold and its TBP-interacting region are similar in TFIID, whereas Taf5 and the Taf6 HEAT domain adopt distinct conformations. Taf12 and Spt20 form flexible connections to the Tra1 module, whereas Sgf73 tethers the DUB module. Binding of a nucleosome to SAGA displaces the HAT and DUB modules from the core-module surface, allowing the DUB module to bind one face of an ubiquitinated nucleosome. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10415.map.gz emd_10415.map.gz | 14.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10415-v30.xml emd-10415-v30.xml emd-10415.xml emd-10415.xml | 33.9 KB 33.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10415_fsc.xml emd_10415_fsc.xml | 14.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_10415.png emd_10415.png | 150.7 KB | ||
| Filedesc metadata |  emd-10415.cif.gz emd-10415.cif.gz | 8.3 KB | ||
| Others |  emd_10415_half_map_1.map.gz emd_10415_half_map_1.map.gz emd_10415_half_map_2.map.gz emd_10415_half_map_2.map.gz | 193.9 MB 194 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10415 http://ftp.pdbj.org/pub/emdb/structures/EMD-10415 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10415 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10415 | HTTPS FTP |
-Related structure data
| Related structure data |  6t9lMC  6t9iC  6t9jC  6t9kC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10415.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10415.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | postprocess masked map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.37 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
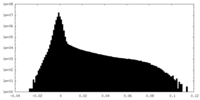
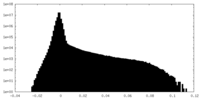
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: half map 1
| File | emd_10415_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
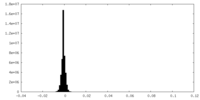
| Density Histograms |
-Half map: #1
| File | emd_10415_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
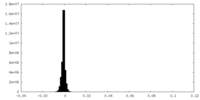
| Density Histograms |
- Sample components
Sample components
+Entire : SAGA DUB-ubiqitinated nucleosome complex
+Supramolecule #1: SAGA DUB-ubiqitinated nucleosome complex
+Supramolecule #2: nucleosome
+Supramolecule #3: SAGA DUB module
+Supramolecule #4: DNA
+Supramolecule #5: Ubiquitin carboxyl-terminal hydrolase 8
+Supramolecule #6: ubiquitin
+Macromolecule #1: Histone H3.2
+Macromolecule #2: Histone H4
+Macromolecule #3: Histone H2A
+Macromolecule #4: Histone H2B 1.1
+Macromolecule #5: Histone H2B
+Macromolecule #8: Ubiquitin carboxyl-terminal hydrolase 8
+Macromolecule #9: Transcription and mRNA export factor SUS1
+Macromolecule #10: SAGA-associated factor 11
+Macromolecule #11: SAGA-associated factor 73
+Macromolecule #12: Polyubiquitin-C
+Macromolecule #6: Widom601 DNA (145-MER)
+Macromolecule #7: Widom601 DNA (145-MER)
+Macromolecule #13: ZINC ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: Solution were made from stock solution | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: blot for 4 seconds before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average exposure time: 9.0 sec. / Average electron dose: 44.17 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)