+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: SASBDB / ID: SASDCP3 |
|---|---|
 試料 試料 | Proline utilization A from Bdellovibrio bacteriovorus
|
| 機能・相同性 |  機能・相同性情報 機能・相同性情報proline dehydrogenase activity / L-glutamate gamma-semialdehyde dehydrogenase / L-glutamate gamma-semialdehyde dehydrogenase activity / L-proline catabolic process to L-glutamate / cytoplasmic side of plasma membrane / DNA-binding transcription factor activity 類似検索 - 分子機能 |
| 生物種 |  Bdellovibrio bacteriovorus (strain ATCC 15356 / DSM 50701 / NCIB 9529 / HD100) (バクテリア) Bdellovibrio bacteriovorus (strain ATCC 15356 / DSM 50701 / NCIB 9529 / HD100) (バクテリア) |
 引用 引用 |  ジャーナル: FEBS J / 年: 2017 ジャーナル: FEBS J / 年: 2017タイトル: Biophysical investigation of type A PutAs reveals a conserved core oligomeric structure. 著者: David A Korasick / Harkewal Singh / Travis A Pemberton / Min Luo / Richa Dhatwalia / John J Tanner /  要旨: Many enzymes form homooligomers, yet the functional significance of self-association is seldom obvious. Herein, we examine the connection between oligomerization and catalytic function for proline ...Many enzymes form homooligomers, yet the functional significance of self-association is seldom obvious. Herein, we examine the connection between oligomerization and catalytic function for proline utilization A (PutA) enzymes. PutAs are bifunctional enzymes that catalyze both reactions of proline catabolism. Type A PutAs are the smallest members of the family, possessing a minimal domain architecture consisting of N-terminal proline dehydrogenase and C-terminal l-glutamate-γ-semialdehyde dehydrogenase modules. Type A PutAs form domain-swapped dimers, and in one case (Bradyrhizobium japonicum PutA), two of the dimers assemble into a ring-shaped tetramer. Whereas the dimer has a clear role in substrate channeling, the functional significance of the tetramer is unknown. To address this question, we performed structural studies of four-type A PutAs from two clades of the PutA tree. The crystal structure of Bdellovibrio bacteriovorus PutA covalently inactivated by N-propargylglycine revealed a fold and substrate-channeling tunnel similar to other PutAs. Small-angle X-ray scattering (SAXS) and analytical ultracentrifugation indicated that Bdellovibrio PutA is dimeric in solution, in contrast to the prediction from crystal packing of a stable tetrameric assembly. SAXS studies of two other type A PutAs from separate clades also suggested that the dimer predominates in solution. To assess whether the tetramer of B. japonicum PutA is necessary for catalytic function, a hot spot disruption mutant that cleanly produces dimeric protein was generated. The dimeric variant exhibited kinetic parameters similar to the wild-type enzyme. These results implicate the domain-swapped dimer as the core structural and functional unit of type A PutAs. ENZYMES: Proline dehydrogenase (EC 1.5.5.2); l-glutamate-γ-semialdehyde dehydrogenase (EC 1.2.1.88). DATABASES: The atomic coordinates and structure factor amplitudes have been deposited in the Protein Data Bank under accession number 5UR2. The SAXS data have been deposited in the SASBDB under the ...DATABASES: The atomic coordinates and structure factor amplitudes have been deposited in the Protein Data Bank under accession number 5UR2. The SAXS data have been deposited in the SASBDB under the following accession codes: SASDCP3 (BbPutA), SASDCQ3 (DvPutA 1.5 mg·mL ), SASDCX3 (DvPutA 3.0 mg·mL ), SASDCY3 (DvPutA 4.5 mg·mL ), SASDCR3 (LpPutA 3.0 mg·mL ), SASDCV3 (LpPutA 5.0 mg·mL ), SASDCW3 (LpPutA 8.0 mg·mL ), SASDCS3 (BjPutA 2.3 mg·mL ), SASDCT3 (BjPutA 4.7 mg·mL ), SASDCU3 (BjPutA 7.0 mg·mL ), SASDCZ3 (R51E 2.3 mg·mL ), SASDC24 (R51E 4.7 mg·mL ), SASDC34 (R51E 7.0 mg·mL ). |
 登録者 登録者 |
|
- 構造の表示
構造の表示
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-モデル
| モデル #1419 |  タイプ: dummy / ダミー原子の半径: 2.90 A / 対称性: P2 / カイ2乗値: 1.168  Omokage検索でこの集合体の類似形状データを探す (詳細) Omokage検索でこの集合体の類似形状データを探す (詳細) |
|---|---|
| モデル #1420 |  タイプ: dummy / ソフトウェア: (5.0 r6293) / ダミー原子の半径: 4.25 A / カイ2乗値: 1.168  Omokage検索でこの集合体の類似形状データを探す (詳細) Omokage検索でこの集合体の類似形状データを探す (詳細) |
- 試料
試料
 試料 試料 | 名称: Proline utilization A from Bdellovibrio bacteriovorus |
|---|---|
| バッファ | 名称: 50 mM Tris, 125 mM NaCl, 1 mM EDTA, and 1 mM tris(3-hydroxypropyl)phosphine (THP) at pH 7.5, pH: 7.5 |
| 要素 #678 | タイプ: protein / 記述: Bifunctional protein PutA / 分子量: 109.504 / 分子数: 2 由来: Bdellovibrio bacteriovorus (strain ATCC 15356 / DSM 50701 / NCIB 9529 / HD100) 参照: UniProt: Q6MNK1 配列: GHMNDIQSQI VSRGEEILKR MESQSKASIF SKDFWYGSIM EWSMKNEKFK TNMFRFVDVL PSINSGDEVA RHLKEYFSED GGTLPPVFNV GLGLGSLAPG LMAGAIKKNV MGMAKMFITG ESPDEALPVL KKARKNKMTF TVDILGEATL SEKEAQDYSN KYMELVTWLA ...配列: GHMNDIQSQI VSRGEEILKR MESQSKASIF SKDFWYGSIM EWSMKNEKFK TNMFRFVDVL PSINSGDEVA RHLKEYFSED GGTLPPVFNV GLGLGSLAPG LMAGAIKKNV MGMAKMFITG ESPDEALPVL KKARKNKMTF TVDILGEATL SEKEAQDYSN KYMELVTWLA KDAEKWDEVP QIDRDHEGAL PKVNVSVKMT ALYSQIKDAA WDESKKILKD RLRPVFRLGM EKGVFVNLDM EQYSVKHLTL EVFTELINEP EFKNYKFFGI VIQAYLRDSF EDVKSLTEFA QKRGTPFWVR LVKGAYWDYE TIEAEQRGWP VPVYTNKAES DANYELCAKY LLENIKFIRP AFASHNVRTL AACMLYAEKL NIPKEALEFQ MLYGMAEPIK KTIVDMGYRM REYAPVGELI PGMAYLVRRL LENTSNESWL RGKFADNKSM AELLKDPAQG LTPTSPVIPK KPGKFYNEPL LDFAVKADRE KMLKALAEAK ASLPVNVNIV INNKELQSGK IFDRVNPSQS DQIVGKIQMA TTEQAEQAMQ AAQTAYKTWK NVPCEQRAAL VDKLADIMTR DRFKLIATQV LEVGKPWAEA DGDIGEAIDF CRYYARHMRE LQKPLRVGGL PGELSHYIYK SRGVTAVIAP WNFPLAILAG MVTAAAVAGN TVVMKPAEQS TVVAWGLMKM IQEAGFPQGV INFLPGYGEE VGEYIVNHKY TTTIAFTGSK AVGLHIMNRA AVVQPGQQHV KRCIIEMGGK NAVIIDNDAD LDEAVDGVIY SAFGFSGQKC SAASRVIVLD EVYDRFVDRL VETAKSIEIH PAENPKAYMG PVVDKEAYDR ILGTIAEAEK NHKLLFKGSV PGGGFFAPPT IFGDVPGDAK LAQAEIFGPV VAVIRAKNLD QALDIANSTE YALTGGVFSR SPANINRVKE ELEVGNLYVN RGITGAMVDR HPFGGFKMSG IGSKTGGPDY LKQYMEPACV TENTLRRGFA PAEE |
-実験情報
| ビーム | 設備名称: Advanced Light Source (ALS) 12.3.1 (SIBYLS) / 地域: Berkeley, CA / 国: USA  / 線源: X-ray synchrotron / 線源: X-ray synchrotron | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 検出器 | 名称: MAR 165 CCD | |||||||||||||||||||||||||||||||||
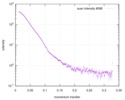
| スキャン | 測定日: 2012年6月8日 / 単位: 1/A /
| |||||||||||||||||||||||||||||||||
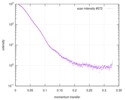
| 距離分布関数 P(R) |
| |||||||||||||||||||||||||||||||||
| 結果 |
|
 ムービー
ムービー コントローラー
コントローラー


 SASDCP3
SASDCP3