[English] 日本語
 Yorodumi
Yorodumi- EMDB-9512: Cryo-EM map of the human 26S proteasome at 3.5A resolution with C... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9512 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM map of the human 26S proteasome at 3.5A resolution with C2 symmetry | ||||||||||||
 Map data Map data | with C2 symmetry | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | protein complex / human proteasome / HYDROLASE | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationImpaired BRCA2 translocation to the nucleus / Impaired BRCA2 binding to SEM1 (DSS1) / thyrotropin-releasing hormone receptor binding / nuclear proteasome complex / host-mediated perturbation of viral transcription / positive regulation of inclusion body assembly / Hydrolases; Acting on peptide bonds (peptidases); Omega peptidases / integrator complex / proteasome accessory complex / meiosis I ...Impaired BRCA2 translocation to the nucleus / Impaired BRCA2 binding to SEM1 (DSS1) / thyrotropin-releasing hormone receptor binding / nuclear proteasome complex / host-mediated perturbation of viral transcription / positive regulation of inclusion body assembly / Hydrolases; Acting on peptide bonds (peptidases); Omega peptidases / integrator complex / proteasome accessory complex / meiosis I / purine ribonucleoside triphosphate binding / proteasome regulatory particle / cytosolic proteasome complex / positive regulation of proteasomal protein catabolic process / proteasome-activating activity / proteasome regulatory particle, lid subcomplex / proteasome regulatory particle, base subcomplex / protein K63-linked deubiquitination / negative regulation of programmed cell death / metal-dependent deubiquitinase activity / Regulation of ornithine decarboxylase (ODC) / Proteasome assembly / Cross-presentation of soluble exogenous antigens (endosomes) / proteasome core complex / Homologous DNA Pairing and Strand Exchange / Defective homologous recombination repair (HRR) due to BRCA1 loss of function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA1 binding function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA2/RAD51/RAD51C binding function / Resolution of D-loop Structures through Synthesis-Dependent Strand Annealing (SDSA) / Somitogenesis / K63-linked deubiquitinase activity / Resolution of D-loop Structures through Holliday Junction Intermediates / Impaired BRCA2 binding to RAD51 / proteasome binding / transcription factor binding / regulation of protein catabolic process / myofibril / proteasome storage granule / Presynaptic phase of homologous DNA pairing and strand exchange / general transcription initiation factor binding / positive regulation of RNA polymerase II transcription preinitiation complex assembly / polyubiquitin modification-dependent protein binding / protein deubiquitination / blastocyst development / immune system process / NF-kappaB binding / proteasome endopeptidase complex / proteasome core complex, beta-subunit complex / endopeptidase activator activity / proteasome assembly / threonine-type endopeptidase activity / mRNA export from nucleus / proteasome core complex, alpha-subunit complex / SARS-CoV-1 targets host intracellular signalling and regulatory pathways / enzyme regulator activity / ERAD pathway / regulation of proteasomal protein catabolic process / inclusion body / proteasome complex / TBP-class protein binding / : / sarcomere / Regulation of activated PAK-2p34 by proteasome mediated degradation / Autodegradation of Cdh1 by Cdh1:APC/C / APC/C:Cdc20 mediated degradation of Securin / stem cell differentiation / N-glycan trimming in the ER and Calnexin/Calreticulin cycle / Asymmetric localization of PCP proteins / Ubiquitin-dependent degradation of Cyclin D / SCF-beta-TrCP mediated degradation of Emi1 / NIK-->noncanonical NF-kB signaling / TNFR2 non-canonical NF-kB pathway / AUF1 (hnRNP D0) binds and destabilizes mRNA / Assembly of the pre-replicative complex / Vpu mediated degradation of CD4 / Ubiquitin-Mediated Degradation of Phosphorylated Cdc25A / negative regulation of inflammatory response to antigenic stimulus / Degradation of DVL / P-body / Cdc20:Phospho-APC/C mediated degradation of Cyclin A / Dectin-1 mediated noncanonical NF-kB signaling / Degradation of AXIN / lipopolysaccharide binding / Hh mutants are degraded by ERAD / Activation of NF-kappaB in B cells / G2/M Checkpoints / Degradation of GLI1 by the proteasome / Hedgehog ligand biogenesis / Defective CFTR causes cystic fibrosis / Autodegradation of the E3 ubiquitin ligase COP1 / Regulation of RUNX3 expression and activity / GSK3B and BTRC:CUL1-mediated-degradation of NFE2L2 / Negative regulation of NOTCH4 signaling / Hedgehog 'on' state / Vif-mediated degradation of APOBEC3G / APC/C:Cdh1 mediated degradation of Cdc20 and other APC/C:Cdh1 targeted proteins in late mitosis/early G1 / double-strand break repair via homologous recombination / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / Degradation of GLI2 by the proteasome / GLI3 is processed to GLI3R by the proteasome Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | ||||||||||||
 Authors Authors | Huang XL / Luan B / Wu JP / Shi YG | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2016 Journal: Nat Struct Mol Biol / Year: 2016Title: An atomic structure of the human 26S proteasome. Authors: Xiuliang Huang / Bai Luan / Jianping Wu / Yigong Shi /  Abstract: We report the cryo-EM structure of the human 26S proteasome at an average resolution of 3.5 Å, allowing atomic modeling of 28 subunits in the core particle (CP) and 18 subunits in the regulatory ...We report the cryo-EM structure of the human 26S proteasome at an average resolution of 3.5 Å, allowing atomic modeling of 28 subunits in the core particle (CP) and 18 subunits in the regulatory particle (RP). The C-terminal residues of Rpt3 and Rpt5 subunits in the RP can be seen inserted into surface pockets formed between adjacent α subunits in the CP. Each of the six Rpt subunits contains a bound nucleotide, and the central gate of the CP α-ring is closed despite RP association. The six pore 1 loops in the Rpt ring are arranged similarly to a spiral staircase along the axial channel of substrate transport, which is constricted by the pore 2 loops. We also determined the cryo-EM structure of the human proteasome bound to the deubiquitinating enzyme USP14 at 4.35-Å resolution. Together, our structures provide a framework for mechanistic understanding of eukaryotic proteasome function. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9512.map.gz emd_9512.map.gz | 478.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9512-v30.xml emd-9512-v30.xml emd-9512.xml emd-9512.xml | 53.1 KB 53.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_9512.png emd_9512.png | 32.5 KB | ||
| Filedesc metadata |  emd-9512.cif.gz emd-9512.cif.gz | 13.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9512 http://ftp.pdbj.org/pub/emdb/structures/EMD-9512 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9512 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9512 | HTTPS FTP |
-Related structure data
| Related structure data |  5gjrMC  9507C  9508C  9509C  9510C  9511C  5gjqC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9512.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9512.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | with C2 symmetry | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
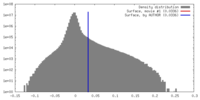
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : human 26S proteasome
+Supramolecule #1: human 26S proteasome
+Macromolecule #1: 26S protease regulatory subunit 4
+Macromolecule #2: 26S protease regulatory subunit 7
+Macromolecule #3: 26S protease regulatory subunit 10B
+Macromolecule #4: 26S protease regulatory subunit 6A
+Macromolecule #5: 26S protease regulatory subunit 8
+Macromolecule #6: 26S protease regulatory subunit 6B
+Macromolecule #7: 26S proteasome non-ATPase regulatory subunit 1
+Macromolecule #8: 26S proteasome non-ATPase regulatory subunit 13
+Macromolecule #9: 26S proteasome non-ATPase regulatory subunit 12
+Macromolecule #10: 26S proteasome non-ATPase regulatory subunit 11
+Macromolecule #11: 26S proteasome non-ATPase regulatory subunit 6
+Macromolecule #12: 26S proteasome non-ATPase regulatory subunit 3
+Macromolecule #13: 26S proteasome non-ATPase regulatory subunit 8
+Macromolecule #14: 26S proteasome non-ATPase regulatory subunit 7
+Macromolecule #15: 26S proteasome non-ATPase regulatory subunit 14
+Macromolecule #16: 26S proteasome non-ATPase regulatory subunit 4
+Macromolecule #17: 26S proteasome complex subunit DSS1
+Macromolecule #18: 26S proteasome non-ATPase regulatory subunit 2
+Macromolecule #19: Proteasome subunit alpha type-6
+Macromolecule #20: Proteasome subunit alpha type-2
+Macromolecule #21: Proteasome subunit alpha type-4
+Macromolecule #22: Proteasome subunit alpha type-7
+Macromolecule #23: Proteasome subunit alpha type-5
+Macromolecule #24: Proteasome subunit alpha type-1
+Macromolecule #25: Proteasome subunit alpha type-3
+Macromolecule #26: Proteasome subunit beta type-6
+Macromolecule #27: Proteasome subunit beta type-7
+Macromolecule #28: Proteasome subunit beta type-3
+Macromolecule #29: Proteasome subunit beta type-2
+Macromolecule #30: Proteasome subunit beta type-5
+Macromolecule #31: Proteasome subunit beta type-1
+Macromolecule #32: Proteasome subunit beta type-4
+Macromolecule #33: ADENOSINE-5'-DIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Material: COPPER / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 3 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV / Details: blot for 2 seconds before plunging. | ||||||||||||
| Details | This sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 70.0 K |
| Details | Preliminary grid screening was performed manually |
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Frames/image: 1-26 / Average exposure time: 1.6 sec. / Average electron dose: 37.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 0.0026000000000000003 µm / Calibrated defocus min: 0.0016 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)