+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | T cell receptor delta 2 gamma 9 with F283A, F290A, and F291A | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | immune / receptor / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationMHC protein binding / regulation of lymphocyte apoptotic process / gamma-delta T cell receptor complex / Fc-gamma receptor III complex / T cell anergy / positive regulation of T cell anergy / positive regulation of cell-cell adhesion mediated by integrin / gamma-delta T cell activation / CD4-positive, alpha-beta T cell proliferation / negative thymic T cell selection ...MHC protein binding / regulation of lymphocyte apoptotic process / gamma-delta T cell receptor complex / Fc-gamma receptor III complex / T cell anergy / positive regulation of T cell anergy / positive regulation of cell-cell adhesion mediated by integrin / gamma-delta T cell activation / CD4-positive, alpha-beta T cell proliferation / negative thymic T cell selection / Fc-gamma receptor signaling pathway / positive regulation of CD4-positive, alpha-beta T cell proliferation / alpha-beta T cell receptor complex / positive regulation of protein localization to cell surface / positive thymic T cell selection / Nef and signal transduction / signal complex assembly / positive regulation of cell-matrix adhesion / T cell receptor complex / smoothened signaling pathway / establishment or maintenance of cell polarity / small molecule binding / positive regulation of interleukin-4 production / Translocation of ZAP-70 to Immunological synapse / Phosphorylation of CD3 and TCR zeta chains / dendrite development / protein complex oligomerization / alpha-beta T cell activation / FCGR activation / Generation of second messenger molecules / immunological synapse / PD-1 signaling / Role of phospholipids in phagocytosis / T cell receptor binding / cell surface receptor protein tyrosine kinase signaling pathway / positive regulation of T cell proliferation / negative regulation of smoothened signaling pathway / T cell costimulation / positive regulation of interleukin-2 production / positive regulation of calcium-mediated signaling / protein tyrosine kinase binding / FCGR3A-mediated IL10 synthesis / cerebellum development / T cell activation / apoptotic signaling pathway / FCGR3A-mediated phagocytosis / calcium-mediated signaling / clathrin-coated endocytic vesicle membrane / Regulation of actin dynamics for phagocytic cup formation / SH3 domain binding / positive regulation of type II interferon production / peptide antigen binding / positive regulation of peptidyl-tyrosine phosphorylation / Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell / transmembrane signaling receptor activity / cell-cell junction / protein transport / Cargo recognition for clathrin-mediated endocytosis / Downstream TCR signaling / signaling receptor complex adaptor activity / Clathrin-mediated endocytosis / T cell receptor signaling pathway / cell body / protein-containing complex assembly / regulation of apoptotic process / adaptive immune response / dendritic spine / cell surface receptor signaling pathway / protein heterodimerization activity / G protein-coupled receptor signaling pathway / external side of plasma membrane / negative regulation of gene expression / innate immune response / positive regulation of gene expression / protein kinase binding / Golgi apparatus / endoplasmic reticulum / protein homodimerization activity / identical protein binding / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Xin W / Huang B / Chi X / Liu Y / Xu M / Zhang Y / Li X / Su Q / Zhou Q | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Structures of human γδ T cell receptor-CD3 complex. Authors: Weizhi Xin / Bangdong Huang / Ximin Chi / Yuehua Liu / Mengjiao Xu / Yuanyuan Zhang / Xu Li / Qiang Su / Qiang Zhou /  Abstract: Gamma delta (γδ) T cells, a unique T cell subgroup, are crucial in various immune responses and immunopathology. The γδ T cell receptor (TCR), which is generated by γδ T cells, recognizes a ...Gamma delta (γδ) T cells, a unique T cell subgroup, are crucial in various immune responses and immunopathology. The γδ T cell receptor (TCR), which is generated by γδ T cells, recognizes a diverse range of antigens independently of the major histocompatibility complex. The γδ TCR associates with CD3 subunits, initiating T cell activation and holding great potential in immunotherapy. Here we report the structures of two prototypical human Vγ9Vδ2 and Vγ5Vδ1 TCR-CD3 complexes, revealing two distinct assembly mechanisms that depend on Vγ usage. The Vγ9Vδ2 TCR-CD3 complex is monomeric, with considerable conformational flexibility in the TCRγ-TCRδ extracellular domain and connecting peptides. The length of the connecting peptides regulates the ligand association and T cell activation. A cholesterol-like molecule wedges into the transmembrane region, exerting an inhibitory role in TCR signalling. The Vγ5Vδ1 TCR-CD3 complex displays a dimeric architecture, whereby two protomers nestle back to back through the Vγ5 domains of the TCR extracellular domains. Our biochemical and biophysical assays further corroborate the dimeric structure. Importantly, the dimeric form of the Vγ5Vδ1 TCR is essential for T cell activation. These findings reveal organizing principles of the γδ TCR-CD3 complex, providing insights into the unique properties of γδ TCR and facilitating immunotherapeutic interventions. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37914.map.gz emd_37914.map.gz | 27.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37914-v30.xml emd-37914-v30.xml emd-37914.xml emd-37914.xml | 20.4 KB 20.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_37914.png emd_37914.png | 50.4 KB | ||
| Filedesc metadata |  emd-37914.cif.gz emd-37914.cif.gz | 6.5 KB | ||
| Others |  emd_37914_half_map_1.map.gz emd_37914_half_map_1.map.gz emd_37914_half_map_2.map.gz emd_37914_half_map_2.map.gz | 23.4 MB 23.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37914 http://ftp.pdbj.org/pub/emdb/structures/EMD-37914 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37914 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37914 | HTTPS FTP |
-Validation report
| Summary document |  emd_37914_validation.pdf.gz emd_37914_validation.pdf.gz | 737 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_37914_full_validation.pdf.gz emd_37914_full_validation.pdf.gz | 736.6 KB | Display | |
| Data in XML |  emd_37914_validation.xml.gz emd_37914_validation.xml.gz | 10.4 KB | Display | |
| Data in CIF |  emd_37914_validation.cif.gz emd_37914_validation.cif.gz | 12.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37914 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37914 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37914 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37914 | HTTPS FTP |
-Related structure data
| Related structure data |  8wy0MC  8jbvC  8jc0C  8jcbC  8wxeC  8wyiC  8yc0C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_37914.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37914.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0773 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_37914_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
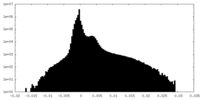
| Projections & Slices |
| ||||||||||||
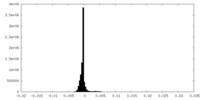
| Density Histograms |
-Half map: #1
| File | emd_37914_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
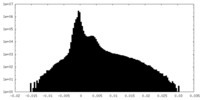
| Projections & Slices |
| ||||||||||||
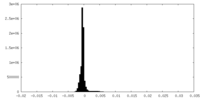
| Density Histograms |
- Sample components
Sample components
-Entire : T cell receptor delta 2 gamma 9 with F283A, F290A, F291A
| Entire | Name: T cell receptor delta 2 gamma 9 with F283A, F290A, F291A |
|---|---|
| Components |
|
-Supramolecule #1: T cell receptor delta 2 gamma 9 with F283A, F290A, F291A
| Supramolecule | Name: T cell receptor delta 2 gamma 9 with F283A, F290A, F291A type: cell / ID: 1 / Parent: 0 / Macromolecule list: #1-#6 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: T-cell surface glycoprotein CD3 zeta chain
| Macromolecule | Name: T-cell surface glycoprotein CD3 zeta chain / type: protein_or_peptide / ID: 1 / Details: 168-195 Twin-Strep tag / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 21.809695 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MKWKALFTAA ILQAQLPITE AQSFGLLDPK LCYLLDGILF IYGVILTALF LRVKFSRSAD APAYQQGQNQ LYNELNLGRR EEYDVLDKR RGRDPEMGGK PQRRKNPQEG LYNELQKDKM AEAYSEIGMK GERRRGKGHD GLYQGLSTAT KDTYDALHMQ A LPPRAAAW ...String: MKWKALFTAA ILQAQLPITE AQSFGLLDPK LCYLLDGILF IYGVILTALF LRVKFSRSAD APAYQQGQNQ LYNELNLGRR EEYDVLDKR RGRDPEMGGK PQRRKNPQEG LYNELQKDKM AEAYSEIGMK GERRRGKGHD GLYQGLSTAT KDTYDALHMQ A LPPRAAAW SHPQFEKGGG SGGGSGGSAW SHPQFEK UniProtKB: T-cell surface glycoprotein CD3 zeta chain |
-Macromolecule #2: T-cell surface glycoprotein CD3 delta chain
| Macromolecule | Name: T-cell surface glycoprotein CD3 delta chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 18.949537 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MEHSTFLSGL VLATLLSQVS PFKIPIEELE DRVFVNCNTS ITWVEGTVGT LLSDITRLDL GKRILDPRGI YRCNGTDIYK DKESTVQVH YRMCQSCVEL DPATVAGIIV TDVIATLLLA LGVFCFAGHE TGRLSGAADT QALLRNDQVY QPLRDRDDAQ Y SHLGGNWA RNK UniProtKB: T-cell surface glycoprotein CD3 delta chain |
-Macromolecule #3: T-cell surface glycoprotein CD3 epsilon chain
| Macromolecule | Name: T-cell surface glycoprotein CD3 epsilon chain / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.174227 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MQSGTHWRVL GLCLLSVGVW GQDGNEEMGG ITQTPYKVSI SGTTVILTCP QYPGSEILWQ HNDKNIGGDE DDKNIGSDED HLSLKEFSE LEQSGYYVCY PRGSKPEDAN FYLYLRARVC ENCMEMDVMS VATIVIVDIC ITGGLLLLVY YWSKNRKAKA K PVTRGAGA ...String: MQSGTHWRVL GLCLLSVGVW GQDGNEEMGG ITQTPYKVSI SGTTVILTCP QYPGSEILWQ HNDKNIGGDE DDKNIGSDED HLSLKEFSE LEQSGYYVCY PRGSKPEDAN FYLYLRARVC ENCMEMDVMS VATIVIVDIC ITGGLLLLVY YWSKNRKAKA K PVTRGAGA GGRQRGQNKE RPPPVPNPDY EPIRKGQRDL YSGLNQRRI UniProtKB: T-cell surface glycoprotein CD3 epsilon chain |
-Macromolecule #4: T-cell surface glycoprotein CD3 gamma chain
| Macromolecule | Name: T-cell surface glycoprotein CD3 gamma chain / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 20.493457 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MEQGKGLAVL ILAIILLQGT LAQSIKGNHL VKVYDYQEDG SVLLTCDAEA KNITWFKDGK MIGFLTEDKK KWNLGSNAKD PRGMYQCKG SQNKSKPLQV YYRMCQNCIE LNAATISGFL FAEIVSIFVL AVGVYFIAGQ DGVRQSRASD KQTLLPNDQL Y QPLKDRED DQYSHLQGNQ LRRN UniProtKB: T-cell surface glycoprotein CD3 gamma chain |
-Macromolecule #5: Signal peptide,flag tag,T cell receptor delta variable 2,T cell r...
| Macromolecule | Name: Signal peptide,flag tag,T cell receptor delta variable 2,T cell receptor delta constant type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 34.331457 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDMRVPAQLL GLLLLWLSGA RCMDYKDDDD KGGSETGAIE LVPEHQTVPV SIGVPATLRC SMKGEAIGNY YINWYRKTQG NTMTFIYRE KDIYGPGFKD NFQGDIDIAK NLAVLKILAP SERDEGSYYC ACDTLGMGGE YTDKLIFGKG TRVTVEPRSQ P HTKPSVFV ...String: MDMRVPAQLL GLLLLWLSGA RCMDYKDDDD KGGSETGAIE LVPEHQTVPV SIGVPATLRC SMKGEAIGNY YINWYRKTQG NTMTFIYRE KDIYGPGFKD NFQGDIDIAK NLAVLKILAP SERDEGSYYC ACDTLGMGGE YTDKLIFGKG TRVTVEPRSQ P HTKPSVFV MKNGTNVACL VKEFYPKDIR INLVSSKKIT EFDPAIVISP SGKYNAVKLG KYEDSNSVTC SVQHDNKTVH ST DFEVKTD STDHVKPKET ENTKQPSKSC HKPKAIVHTE KVNMMSLTVL GLRMLFAKTV AVNALLTAKL AAL UniProtKB: T cell receptor delta variable 2, T cell receptor delta constant |
-Macromolecule #6: Signal peptide,flag tag,T cell receptor gamma variable 9,T cell r...
| Macromolecule | Name: Signal peptide,flag tag,T cell receptor gamma variable 9,T cell receptor gamma constant 1 type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.406973 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDMRVPAQLL GLLLLWLSGA RCMDYKDDDD KGGSETGAGH LEQPQISSTK TLSKTARLEC VVSGITISAT SVYWYRERPG EVIQFLVSI SYDGTVRKES GIPSGKFEVD RIPETSTSTL TIHNVEKQDI ATYYCALWEA QQELGKKIKV FGPGTKLIIT D KQLDADVS ...String: MDMRVPAQLL GLLLLWLSGA RCMDYKDDDD KGGSETGAGH LEQPQISSTK TLSKTARLEC VVSGITISAT SVYWYRERPG EVIQFLVSI SYDGTVRKES GIPSGKFEVD RIPETSTSTL TIHNVEKQDI ATYYCALWEA QQELGKKIKV FGPGTKLIIT D KQLDADVS PKPTIFLPSI AETKLQKAGT YLCLLEKFFP DVIKIHWQEK KSNTILGSQE GNTMKTNDTY MKFSWLTVPE KS LDKEHRC IVRHENNKNG VDQEIIFPPI KTDVITMDPK DNCSKDANDT LLLQLTNTSA YYMYLLLLLK SVVYFAIITC CLL RRTAFC CNGEKS UniProtKB: T cell receptor gamma variable 9, T cell receptor gamma constant 1 |
-Macromolecule #7: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 7 / Number of copies: 1 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.8 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 294780 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller



































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)