[English] 日本語
 Yorodumi
Yorodumi- EMDB-3374: Cryo-electron microscopy structure of the hexagonal pre-attachmen... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3374 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-electron microscopy structure of the hexagonal pre-attachment T4 baseplate-tail tube complex | |||||||||
 Map data Map data | Cryo-electron microscopy structure of the hexagonal pre-attachment T4 baseplate-tail tube complex. Post-processed with RELION, using auto-masking with initial binarization threshold of 0.011. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | T4 / baseplate-tail tube complex / pre-attachment / bacteriophage / bacterial virus / hexagonal / membrane-piercing / cell attachment / infection | |||||||||
| Function / homology |  Function and homology information Function and homology informationpeptidoglycan beta-N-acetylmuramidase activity / symbiont genome ejection through host cell envelope, contractile tail mechanism / symbiont entry into host cell via disruption of host cell wall peptidoglycan / virus tail, tube / virus tail, baseplate / viral tail assembly / virus tail, fiber / symbiont entry into host cell via disruption of host cell envelope / virus tail / symbiont entry into host ...peptidoglycan beta-N-acetylmuramidase activity / symbiont genome ejection through host cell envelope, contractile tail mechanism / symbiont entry into host cell via disruption of host cell wall peptidoglycan / virus tail, tube / virus tail, baseplate / viral tail assembly / virus tail, fiber / symbiont entry into host cell via disruption of host cell envelope / virus tail / symbiont entry into host / viral release from host cell / peptidoglycan catabolic process / cell wall macromolecule catabolic process / lysozyme / lysozyme activity / killing of cells of another organism / entry receptor-mediated virion attachment to host cell / defense response to bacterium / symbiont entry into host cell / structural molecule activity / metal ion binding / identical protein binding Similarity search - Function | |||||||||
| Biological species |  Enterobacteria phage T4 (virus) Enterobacteria phage T4 (virus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.11 Å | |||||||||
 Authors Authors | Taylor NMI / Guerrero-Ferreira RC / Goldie KN / Stahlberg H / Leiman PG | |||||||||
 Citation Citation |  Journal: Nature / Year: 2016 Journal: Nature / Year: 2016Title: Structure of the T4 baseplate and its function in triggering sheath contraction. Authors: Nicholas M I Taylor / Nikolai S Prokhorov / Ricardo C Guerrero-Ferreira / Mikhail M Shneider / Christopher Browning / Kenneth N Goldie / Henning Stahlberg / Petr G Leiman /   Abstract: Several systems, including contractile tail bacteriophages, the type VI secretion system and R-type pyocins, use a multiprotein tubular apparatus to attach to and penetrate host cell membranes. This ...Several systems, including contractile tail bacteriophages, the type VI secretion system and R-type pyocins, use a multiprotein tubular apparatus to attach to and penetrate host cell membranes. This macromolecular machine resembles a stretched, coiled spring (or sheath) wound around a rigid tube with a spike-shaped protein at its tip. A baseplate structure, which is arguably the most complex part of this assembly, relays the contraction signal to the sheath. Here we present the atomic structure of the approximately 6-megadalton bacteriophage T4 baseplate in its pre- and post-host attachment states and explain the events that lead to sheath contraction in atomic detail. We establish the identity and function of a minimal set of components that is conserved in all contractile injection systems and show that the triggering mechanism is universally conserved. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3374.map.gz emd_3374.map.gz | 38.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3374-v30.xml emd-3374-v30.xml emd-3374.xml emd-3374.xml | 10.5 KB 10.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3374_fsc.xml emd_3374_fsc.xml | 16.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_3374.png emd_3374.png | 149.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3374 http://ftp.pdbj.org/pub/emdb/structures/EMD-3374 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3374 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3374 | HTTPS FTP |
-Related structure data
| Related structure data |  5iv5MC  3392C  3393C  3394C  3395C  3396C  3397C  5iv7C  5iw9C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3374.map.gz / Format: CCP4 / Size: 412 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3374.map.gz / Format: CCP4 / Size: 412 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-electron microscopy structure of the hexagonal pre-attachment T4 baseplate-tail tube complex. Post-processed with RELION, using auto-masking with initial binarization threshold of 0.011. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.326 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
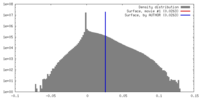
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Hexagonal pre-attachment T4 baseplate-tail tube complex
| Entire | Name: Hexagonal pre-attachment T4 baseplate-tail tube complex |
|---|---|
| Components |
|
-Supramolecule #1000: Hexagonal pre-attachment T4 baseplate-tail tube complex
| Supramolecule | Name: Hexagonal pre-attachment T4 baseplate-tail tube complex type: sample / ID: 1000 Details: In addition to hexagonal pre-attachment baseplate-tail tube complexes, the sample also contained some star-shaped, hubless post-attachment baseplates.The current reconstruction is the ...Details: In addition to hexagonal pre-attachment baseplate-tail tube complexes, the sample also contained some star-shaped, hubless post-attachment baseplates.The current reconstruction is the overall reconstruction of the hexagonal pre-attachment baseplate-tail tube complex. Oligomeric state: (gp3)6(gp5)3(gp5.4)1(gp6)12(gp7)6(gp8)12(gp9)18(gp10)18(gp11)18(gp12)18(gp19)126(gp25)6(gp27)3(gp29)X(gp48)6(gp53)6(gp54)6 Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 8.7 MDa |
-Macromolecule #1: Hexagonal pre-attachment T4 baseplate-tail tube complex
| Macromolecule | Name: Hexagonal pre-attachment T4 baseplate-tail tube complex type: protein_or_peptide / ID: 1 / Number of copies: 1 / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  Enterobacteria phage T4 (virus) / Strain: am18/am23 mutant Enterobacteria phage T4 (virus) / Strain: am18/am23 mutant |
| Molecular weight | Theoretical: 8.7 MDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 50 mM Tris-Hcl pH 8.0, 100 mM NaCl, 8 mM MgSO4 |
| Grid | Details: Quantifoil 300 mesh carbon-coated copper grids glow-discharged for 20 seconds |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV Method: Applied 3.5 ul of sample and blotting 3 seconds before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Average: 80 K |
| Alignment procedure | Legacy - Astigmatism: The astigmatism was corrected at high magnification |
| Specialist optics | Energy filter - Name: Quantum-LS Gatan Image Filter / Energy filter - Lower energy threshold: 0.0 eV / Energy filter - Upper energy threshold: 20.0 eV |
| Date | May 7, 2015 |
| Image recording | Category: CCD / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Number real images: 1621 / Average electron dose: 60 e/Å2 Details: Individual frames were aligned with 2dx_automator. 40 frames were recorded in total, and the 2 first frames were discarded. Bits/pixel: 32 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 37700 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





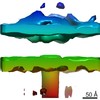








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)