+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2613 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Incomplete pneumolysin oligomers form membrane pores | |||||||||
 Map data Map data | Average of 99 sub-tomogram volumes. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | cholesterol-dependent cytolysin / pneumolysin / proteolipid toroidal pore | |||||||||
| Function / homology |  Function and homology information Function and homology informationcholesterol binding / toxin activity / killing of cells of another organism / host cell plasma membrane / extracellular region / membrane Similarity search - Function | |||||||||
| Biological species | synthetic construct (others) /  | |||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 29.0 Å | |||||||||
 Authors Authors | Sonnen AF-P / Plitzko JM / Gilbert RJC | |||||||||
 Citation Citation |  Journal: Open Biol / Year: 2014 Journal: Open Biol / Year: 2014Title: Incomplete pneumolysin oligomers form membrane pores. Authors: Andreas F-P Sonnen / Jürgen M Plitzko / Robert J C Gilbert /  Abstract: Pneumolysin is a member of the cholesterol-dependent cytolysin (CDC) family of pore-forming proteins that are produced as water-soluble monomers or dimers, bind to target membranes and oligomerize ...Pneumolysin is a member of the cholesterol-dependent cytolysin (CDC) family of pore-forming proteins that are produced as water-soluble monomers or dimers, bind to target membranes and oligomerize into large ring-shaped assemblies comprising approximately 40 subunits and approximately 30 nm across. This pre-pore assembly then refolds to punch a large hole in the lipid bilayer. However, in addition to forming large pores, pneumolysin and other CDCs form smaller lesions characterized by low electrical conductance. Owing to the observation of arc-like (rather than full-ring) oligomers by electron microscopy, it has been hypothesized that smaller oligomers explain smaller functional pores. To investigate whether this is the case, we performed cryo-electron tomography of pneumolysin oligomers on model lipid membranes. We then used sub-tomogram classification and averaging to determine representative membrane-bound low-resolution structures and identified pre-pores versus pores by the presence of membrane within the oligomeric curve. We found pre-pore and pore forms of both complete (ring) and incomplete (arc) oligomers and conclude that arc-shaped oligomeric assemblies of pneumolysin can form pores. As the CDCs are evolutionarily related to the membrane attack complex/perforin family of proteins, which also form variably sized pores, our findings are of relevance to that class of proteins as well. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2613.map.gz emd_2613.map.gz | 3.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2613-v30.xml emd-2613-v30.xml emd-2613.xml emd-2613.xml | 9.3 KB 9.3 KB | Display Display |  EMDB header EMDB header |
| Images |  EMD2613.gif EMD2613.gif | 45.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2613 http://ftp.pdbj.org/pub/emdb/structures/EMD-2613 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2613 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2613 | HTTPS FTP |
-Related structure data
| Related structure data |  2611C  2612C  2614C  2615C  2616C  2617C  2618C  2619C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_2613.map.gz / Format: CCP4 / Size: 4.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2613.map.gz / Format: CCP4 / Size: 4.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Average of 99 sub-tomogram volumes. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.72 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
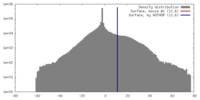
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Pneumolysin prepore formed on a cholesterol-containing liposome
| Entire | Name: Pneumolysin prepore formed on a cholesterol-containing liposome |
|---|---|
| Components |
|
-Supramolecule #1000: Pneumolysin prepore formed on a cholesterol-containing liposome
| Supramolecule | Name: Pneumolysin prepore formed on a cholesterol-containing liposome type: sample / ID: 1000 / Oligomeric state: Full ring oligomer / Number unique components: 2 |
|---|---|
| Molecular weight | Theoretical: 2 MDa |
-Supramolecule #1: synthetic membrane
| Supramolecule | Name: synthetic membrane / type: organelle_or_cellular_component / ID: 1 Details: Liposome formed of a 10:10:1 mixture of phosphatidylcholine:cholesterol:dicetyl phosphate Recombinant expression: No |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
-Macromolecule #1: pneumolysin
| Macromolecule | Name: pneumolysin / type: protein_or_peptide / ID: 1 Details: pneumolysin added to the synthetic membranes spontaneously forms oligomers on their surfaces. Oligomeric state: 40mer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 52 KDa / Theoretical: 52 KDa |
| Recombinant expression | Organism:  |
| Sequence | UniProtKB: Pneumolysin |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 / Details: PBS |
|---|---|
| Grid | Details: C-flat or lacy carbon-coated grids |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 100 K / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Specialist optics | Energy filter - Name: GIF2002 / Energy filter - Lower energy threshold: 0.0 eV / Energy filter - Upper energy threshold: 20.0 eV |
| Date | Jun 1, 2008 |
| Image recording | Category: CCD / Film or detector model: GATAN MULTISCAN |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal defocus max: 8.0 µm / Nominal defocus min: 8.0 µm / Nominal magnification: 64171 |
| Sample stage | Specimen holder: Nitrogen cooled / Specimen holder model: GATAN LIQUID NITROGEN / Tilt series - Axis1 - Min angle: -65 ° / Tilt series - Axis1 - Max angle: 65 ° |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | Subtomograms were selected manually in Bshow and subjected to automatic maximum-likelihood based classification using XMIPP. |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 29.0 Å / Resolution method: OTHER / Software - Name: TOM, Toolbox, Bsoft, XMIPP / Number subtomograms used: 99 |
 Movie
Movie Controller
Controller




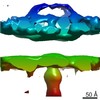



 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)