[English] 日本語
 Yorodumi
Yorodumi- EMDB-12661: Respiratory complex I from Escherichia coli - focused refinement ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12661 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
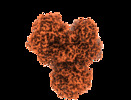
| Title | Respiratory complex I from Escherichia coli - focused refinement of cytoplasmic arm | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationTranslocases; Catalysing the translocation of protons; Linked to oxidoreductase reactions / NADH dehydrogenase (quinone) (non-electrogenic) activity / NADH dehydrogenase complex / cellular respiration / molybdopterin cofactor binding / respiratory chain complex I / NADH dehydrogenase (ubiquinone) activity / quinone binding / ATP synthesis coupled electron transport / proton transmembrane transport ...Translocases; Catalysing the translocation of protons; Linked to oxidoreductase reactions / NADH dehydrogenase (quinone) (non-electrogenic) activity / NADH dehydrogenase complex / cellular respiration / molybdopterin cofactor binding / respiratory chain complex I / NADH dehydrogenase (ubiquinone) activity / quinone binding / ATP synthesis coupled electron transport / proton transmembrane transport / aerobic respiration / respiratory electron transport chain / 2 iron, 2 sulfur cluster binding / NAD binding / FMN binding / 4 iron, 4 sulfur cluster binding / oxidoreductase activity / iron ion binding / metal ion binding / membrane / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.1 Å | |||||||||
 Authors Authors | Kolata P / Efremov RG | |||||||||
| Funding support |  Belgium, 1 items Belgium, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2021 Journal: Elife / Year: 2021Title: Structure of respiratory complex I reconstituted into lipid nanodiscs reveals an uncoupled conformation. Authors: Piotr Kolata / Rouslan G Efremov /  Abstract: Respiratory complex I is a multi-subunit membrane protein complex that reversibly couples NADH oxidation and ubiquinone reduction with proton translocation against transmembrane potential. Complex I ...Respiratory complex I is a multi-subunit membrane protein complex that reversibly couples NADH oxidation and ubiquinone reduction with proton translocation against transmembrane potential. Complex I from is among the best functionally characterized complexes, but its structure remains unknown, hindering further studies to understand the enzyme coupling mechanism. Here, we describe the single particle cryo-electron microscopy (cryo-EM) structure of the entire catalytically active complex I reconstituted into lipid nanodiscs. The structure of this mesophilic bacterial complex I displays highly dynamic connection between the peripheral and membrane domains. The peripheral domain assembly is stabilized by unique terminal extensions and an insertion loop. The membrane domain structure reveals novel dynamic features. Unusual conformation of the conserved interface between the peripheral and membrane domains suggests an uncoupled conformation of the complex. Considering constraints imposed by the structural data, we suggest a new simple hypothetical coupling mechanism for the molecular machine. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12661.map.gz emd_12661.map.gz | 19.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12661-v30.xml emd-12661-v30.xml emd-12661.xml emd-12661.xml | 27.5 KB 27.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12661_fsc.xml emd_12661_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_12661.png emd_12661.png | 178.9 KB | ||
| Others |  emd_12661_half_map_1.map.gz emd_12661_half_map_1.map.gz emd_12661_half_map_2.map.gz emd_12661_half_map_2.map.gz | 104 MB 104.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12661 http://ftp.pdbj.org/pub/emdb/structures/EMD-12661 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12661 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12661 | HTTPS FTP |
-Validation report
| Summary document |  emd_12661_validation.pdf.gz emd_12661_validation.pdf.gz | 393.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_12661_full_validation.pdf.gz emd_12661_full_validation.pdf.gz | 392.8 KB | Display | |
| Data in XML |  emd_12661_validation.xml.gz emd_12661_validation.xml.gz | 15.4 KB | Display | |
| Data in CIF |  emd_12661_validation.cif.gz emd_12661_validation.cif.gz | 18.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12661 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12661 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12661 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12661 | HTTPS FTP |
-Related structure data
| Related structure data |  7nz1MC  7nyhC  7nyrC  7nyuC  7nyvC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12661.map.gz / Format: CCP4 / Size: 21 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12661.map.gz / Format: CCP4 / Size: 21 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.7712 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: #2
| File | emd_12661_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
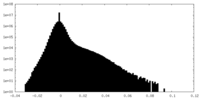
| Density Histograms |
-Half map: #1
| File | emd_12661_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Respiratory complex I from Escherichia coli - focused refinement ...
+Supramolecule #1: Respiratory complex I from Escherichia coli - focused refinement ...
+Macromolecule #1: NADH-quinone oxidoreductase subunit B
+Macromolecule #2: NADH-quinone oxidoreductase subunit C/D
+Macromolecule #3: NADH-quinone oxidoreductase subunit E
+Macromolecule #4: NADH-quinone oxidoreductase subunit F
+Macromolecule #5: NADH-quinone oxidoreductase subunit G
+Macromolecule #6: NADH-quinone oxidoreductase subunit I
+Macromolecule #7: IRON/SULFUR CLUSTER
+Macromolecule #8: FE2/S2 (INORGANIC) CLUSTER
+Macromolecule #9: FLAVIN MONONUCLEOTIDE
+Macromolecule #10: CALCIUM ION
+Macromolecule #11: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 6.8 Component:
Details: The buffer was used for gel filtration of protein reconstituted in lipid nanodiscs | ||||||||||||
| Grid | Model: Quantifoil R0.6/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.028 kPa | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 97 % / Chamber temperature: 296 K / Instrument: GATAN CRYOPLUNGE 3 |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Specialist optics | Energy filter - Name: In-column Omega Filter / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 9122 / Average exposure time: 3.0 sec. / Average electron dose: 64.7 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.55 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 60000 |
| Sample stage | Specimen holder model: JEOL CRYOSPECPORTER / Cooling holder cryogen: NITROGEN |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)