+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10592 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Virion of empty GTA particle | ||||||||||||||||||||||||
 Map data Map data | virion of empty gene transfer agent (GTA) particle, C5 symmetry, 4-times binned | ||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||
 Keywords Keywords | "gene transfer agent" / "virion" / "genome release" / "HK97" / VIRUS | ||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationPhage conserved hypothetical protein / Tail completion protein / Tail completion protein gp17 / Gene transfer agent, major tail protein / : / Phage portal protein, HK97 / Bacteriophage SPP1, head-tail adaptor superfamily / Bacteriophage SPP1, head-tail adaptor / Phage head completion protein / Phage major tail protein TP901-1 ...Phage conserved hypothetical protein / Tail completion protein / Tail completion protein gp17 / Gene transfer agent, major tail protein / : / Phage portal protein, HK97 / Bacteriophage SPP1, head-tail adaptor superfamily / Bacteriophage SPP1, head-tail adaptor / Phage head completion protein / Phage major tail protein TP901-1 / Phage tail tube protein / Bacteriophage/Gene transfer agent portal protein / Phage portal protein / : / Phage capsid / Phage capsid family Similarity search - Domain/homology | ||||||||||||||||||||||||
| Biological species |  Rhodobacter capsulatus SB 1003 (bacteria) Rhodobacter capsulatus SB 1003 (bacteria) | ||||||||||||||||||||||||
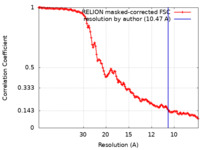
| Method | single particle reconstruction / cryo EM / Resolution: 10.47 Å | ||||||||||||||||||||||||
 Authors Authors | Bardy P / Fuzik T | ||||||||||||||||||||||||
| Funding support |  Czech Republic, 7 items Czech Republic, 7 items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Structure and mechanism of DNA delivery of a gene transfer agent. Authors: Pavol Bárdy / Tibor Füzik / Dominik Hrebík / Roman Pantůček / J Thomas Beatty / Pavel Plevka /   Abstract: Alphaproteobacteria, which are the most abundant microorganisms of temperate oceans, produce phage-like particles called gene transfer agents (GTAs) that mediate lateral gene exchange. However, the ...Alphaproteobacteria, which are the most abundant microorganisms of temperate oceans, produce phage-like particles called gene transfer agents (GTAs) that mediate lateral gene exchange. However, the mechanism by which GTAs deliver DNA into cells is unknown. Here we present the structure of the GTA of Rhodobacter capsulatus (RcGTA) and describe the conformational changes required for its DNA ejection. The structure of RcGTA resembles that of a tailed phage, but it has an oblate head shortened in the direction of the tail axis, which limits its packaging capacity to less than 4,500 base pairs of linear double-stranded DNA. The tail channel of RcGTA contains a trimer of proteins that possess features of both tape measure proteins of long-tailed phages from the family Siphoviridae and tail needle proteins of short-tailed phages from the family Podoviridae. The opening of a constriction within the RcGTA baseplate enables the ejection of DNA into bacterial periplasm. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10592.map.gz emd_10592.map.gz | 99 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10592-v30.xml emd-10592-v30.xml emd-10592.xml emd-10592.xml | 29.7 KB 29.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10592_fsc.xml emd_10592_fsc.xml | 12.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_10592.png emd_10592.png | 65.9 KB | ||
| Filedesc metadata |  emd-10592.cif.gz emd-10592.cif.gz | 7.5 KB | ||
| Others |  emd_10592_additional_1.map.gz emd_10592_additional_1.map.gz | 73.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10592 http://ftp.pdbj.org/pub/emdb/structures/EMD-10592 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10592 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10592 | HTTPS FTP |
-Related structure data
| Related structure data |  6tuiMC  6tb9C  6tbaC  6te8C  6te9C  6teaC  6tebC  6tehC  6to8C  6toaC  6tsuC  6tsvC  6tswC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10592.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10592.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | virion of empty gene transfer agent (GTA) particle, C5 symmetry, 4-times binned | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.252 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
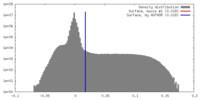
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: virion of empty gene transfer agent (GTA) particle,...
| File | emd_10592_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | virion of empty gene transfer agent (GTA) particle, map assembled from symmetrized subparticles | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Rhodobacter capsulatus DE442
+Supramolecule #1: Rhodobacter capsulatus DE442
+Supramolecule #2: Capsid
+Supramolecule #3: Head spike
+Supramolecule #4: Connector
+Supramolecule #5: Tail
+Macromolecule #1: Phage major capsid protein, HK97 family
+Macromolecule #2: Uncharacterized protein
+Macromolecule #3: Uncharacterized protein
+Macromolecule #4: Tail tube protein Rcc01691
+Macromolecule #5: Adaptor protein Rcc01688
+Macromolecule #6: Portal protein Rcc01684
+Macromolecule #7: Tail terminator protein Rcc01690
+Macromolecule #8: Stopper protein Rcc01689
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 20 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.8 Component:
Details: G-buffer, doi: 10.1016/0003-9861(77)90508-2 | ||||||||||||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 11 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: OTHER | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Average electron dose: 42.75 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -3.0 µm / Nominal defocus min: -1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-6tui: |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)