+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23267 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Bacterial cellulose synthase BcsB with polyalanine BcsA model | ||||||||||||
 Map data Map data | Bacterial cellulose synthase BcsB with polyalanine BcsA model | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | bacterial cellulose / synthase / structural subunit / BIOSYNTHETIC PROTEIN / TRANSFERASE | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationbacterial cellulose biosynthetic process / Biofilm formation / cellulose synthase (UDP-forming) / cellulose synthase (UDP-forming) activity / cellulose biosynthetic process / UDP-alpha-D-glucose metabolic process / hexosyltransferase activity / cyclic-di-GMP binding / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |   | ||||||||||||
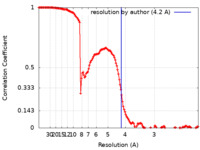
| Method | single particle reconstruction / cryo EM / Resolution: 4.2 Å | ||||||||||||
 Authors Authors | Acheson JF / Zimmer J | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2021 Journal: Nat Struct Mol Biol / Year: 2021Title: Molecular organization of the E. coli cellulose synthase macrocomplex. Authors: Justin F Acheson / Ruoya Ho / Nicolette F Goularte / Lynette Cegelski / Jochen Zimmer /  Abstract: Cellulose is frequently found in communities of sessile bacteria called biofilms. Escherichia coli and other enterobacteriaceae modify cellulose with phosphoethanolamine (pEtN) to promote host tissue ...Cellulose is frequently found in communities of sessile bacteria called biofilms. Escherichia coli and other enterobacteriaceae modify cellulose with phosphoethanolamine (pEtN) to promote host tissue adhesion. The E. coli pEtN cellulose biosynthesis machinery contains the catalytic BcsA-B complex that synthesizes and secretes cellulose, in addition to five other subunits. The membrane-anchored periplasmic BcsG subunit catalyzes pEtN modification. Here we present the structure of the roughly 1 MDa E. coli Bcs complex, consisting of one BcsA enzyme associated with six copies of BcsB, determined by single-particle cryo-electron microscopy. BcsB homo-oligomerizes primarily through interactions between its carbohydrate-binding domains as well as intermolecular beta-sheet formation. The BcsB hexamer creates a half spiral whose open side accommodates two BcsG subunits, directly adjacent to BcsA's periplasmic channel exit. The cytosolic BcsE and BcsQ subunits associate with BcsA's regulatory PilZ domain. The macrocomplex is a fascinating example of cellulose synthase specification. #1:  Journal: Nat.Struct.Mol.Biol. / Year: 2021 Journal: Nat.Struct.Mol.Biol. / Year: 2021Title: Molecular Organization of the E. coli Cellulose Synthase Macrocomplex Authors: Acheson JF / Ho R / Goularte NF / Cegelski L / Zimmer J | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23267.map.gz emd_23267.map.gz | 229.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23267-v30.xml emd-23267-v30.xml emd-23267.xml emd-23267.xml | 18.8 KB 18.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23267_fsc.xml emd_23267_fsc.xml | 14 KB | Display |  FSC data file FSC data file |
| Images |  emd_23267.png emd_23267.png | 51.9 KB | ||
| Masks |  emd_23267_msk_1.map emd_23267_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-23267.cif.gz emd-23267.cif.gz | 7.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23267 http://ftp.pdbj.org/pub/emdb/structures/EMD-23267 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23267 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23267 | HTTPS FTP |
-Related structure data
| Related structure data |  7lbyMC  7l2zC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10627 (Title: Poly-alanine backbone model of E. coli BcsA bound to BcsB EMPIAR-10627 (Title: Poly-alanine backbone model of E. coli BcsA bound to BcsBData size: 3.2 TB Data #1: Unaligned multi-frame micrographs [micrographs - multiframe] Data #2: Unaligned movie frames [micrographs - multiframe] / Data #3: Unaligned movie frames [micrographs - multiframe] / Data #4: Unaligned movie frames [micrographs - multiframe] / Data #5: Unaligned movie frames [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23267.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23267.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Bacterial cellulose synthase BcsB with polyalanine BcsA model | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_23267_msk_1.map emd_23267_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Bacterial cellulose synthase BcsB with BcsA
| Entire | Name: Bacterial cellulose synthase BcsB with BcsA |
|---|---|
| Components |
|
-Supramolecule #1: Bacterial cellulose synthase BcsB with BcsA
| Supramolecule | Name: Bacterial cellulose synthase BcsB with BcsA / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: BcsB monomer with BcsA refined using masked local refinement and signal subtraction from E coli BCS inner-membrane complex projections. |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 180 KDa |
-Macromolecule #1: Cellulose synthase catalytic subunit [UDP-forming]
| Macromolecule | Name: Cellulose synthase catalytic subunit [UDP-forming] / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: cellulose synthase (UDP-forming) |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 101.67125 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSILTRWLLI PPVNARLIGR YRDYRRHGAS AFSATLGCFW MILAWIFIPL EHPRWQRIRA EHKNLYPHIN ASRPRPLDPV RYLIQTCWL LIGASRKETP KPRRRAFSGL QNIRGRYHQW MNELPERVSH KTQHLDEKKE LGHLSAGARR LILGIIVTFS L ILALICVT ...String: GSILTRWLLI PPVNARLIGR YRDYRRHGAS AFSATLGCFW MILAWIFIPL EHPRWQRIRA EHKNLYPHIN ASRPRPLDPV RYLIQTCWL LIGASRKETP KPRRRAFSGL QNIRGRYHQW MNELPERVSH KTQHLDEKKE LGHLSAGARR LILGIIVTFS L ILALICVT QPFNPLAQFI FLMLLWGVAL IVRRMPGRFS ALMLIVLSLT VSCRYIWWRY TSTLNWDDPV SLVCGLILLF AE TYAWIVL VLGYFQVVWP LNRQPVPLPK DMSLWPSVDI FVPTYNEDLN VVKNTIYASL GIDWPKDKLN IWILDDGGRE EFR QFAQNV GVKYIARTTH EHAKAGNINN ALKYAKGEFV SIFDCDHVPT RSFLQMTMGW FLKEKQLAMM QTPHHFFSPD PFER NLGRF RKTPNEGTLF YGLVQDGNDM WDATFFCGSC AVIRRKPLDE IGGIAVETVT EDAHTSLRLH RRGYTSAYMR IPQAA GLAT ESLSAHIGQR IRWARGMVQI FRLDNPLTGK GLKFAQRLCY VNAMFHFLSG IPRLIFLTAP LAFLLLHAYI IYAPAL MIA LFVLPHMIHA SLTNSKIQGK YRHSFWSEIY ETVLAWYIAP PTLVALINPH KGKFNVTAKG GLVEEEYVDW VISRPYI FL VLLNLVGVAV GIWRYFYGPP TEMLTVVVSM VWVFYNLIVL GGAVAVSVES KQVRRSHRVE MTMPAAIARE DGHLFSCT V QDFSDGGLGI KINGQAQILE GQKVNLLLKR GQQEYVFPTQ VARVMGNEVG LKLMPLTTQQ HIDFVQCTFA RADTWALWQ DSYPEDKPLE SLLDILKLGF RGYRHLAEFA PSSVKGIFRV LTSLVSWVVS FIPPRPERSE TAQPSDQALA QQHHHHHHLE HHHHHH UniProtKB: Cellulose synthase catalytic subunit [UDP-forming] |
-Macromolecule #2: Cyclic di-GMP-binding protein
| Macromolecule | Name: Cyclic di-GMP-binding protein / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 86.101289 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKRKLFWICA VAMGMSAFPS FMTQATPATQ PLINAEPAVA AQTEQNPQVG QVMPGVQGAD APVVAQNGPS RDVKLTFAQI APPPGSMVL RGINPNGSIE FGMRSDEVVT KAMLNLEYTP SPSLLPVQSQ LKVYLNDELM GVLPVTKEQL GKKTLAQMPI N PLFISDFN ...String: MKRKLFWICA VAMGMSAFPS FMTQATPATQ PLINAEPAVA AQTEQNPQVG QVMPGVQGAD APVVAQNGPS RDVKLTFAQI APPPGSMVL RGINPNGSIE FGMRSDEVVT KAMLNLEYTP SPSLLPVQSQ LKVYLNDELM GVLPVTKEQL GKKTLAQMPI N PLFISDFN RVRLEFVGHY QDVCEKPAST TLWLDVGRSS GLDLTYQTLN VKNDLSHFPV PFFDPSDNRT NTLPMVFAGA PD VGLQQAS AIVASWFGSR SGWRGQNFPV LYNQLPDRNA IVFATNDKRP DFLRDHPAVK APVIEMINHP QNPYVKLLVV FGR DDKDLL QAAKGIAQGN ILFRGESVVV NEVKPLLPRK PYDAPNWVRT DRPVTFGELK TYEEQLQSSG LEPAAINVSL NLPP DLYLM RSTGIDMDIN YRYTMPPVKD SSRMDISLNN QFLQSFNLSS KQEANRLLLR IPVLQGLLDG KTDVSIPALK LGATN QLRF DFEYMNPMPG GSVDNCITFQ PVQNHVVIGD DSTIDFSKYY HFIPMPDLRA FANAGFPFSR MADLSQTITV MPKAPN EAQ METLLNTVGF IGAQTGFPAI NLTVTDDGST IQGKDADIMI IGGIPDKLKD DKQIDLLVQA TESWVKTPMR QTPFPGI VP DESDRAAETR STLTSSGAMA AVIGFQSPYN DQRSVIALLA DSPRGYEMLN DAVNDSGKRA TMFGSVAVIR ESGINSLR V GDVYYVGHLP WFERVWYALA NHPILLAVLA AISVILLAWV LWRLLRIISR RRLNPDNE UniProtKB: Cyclic di-GMP-binding protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: All buffers made fresh from concentrated stock. A 10% LMNG 2% DMNG 2% CHS solution was made made in milliQ water without buffer. After rocking for one day at room temp the solution was ...Details: All buffers made fresh from concentrated stock. A 10% LMNG 2% DMNG 2% CHS solution was made made in milliQ water without buffer. After rocking for one day at room temp the solution was briefly sonicated to completely dissolve the CHS. | ||||||||||||||||||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: OTHER / Details: 2 drops amylamine added to the chamber | ||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: 3.5 uL applied to c-flat 1.2/1.3 300 mesh grids incubated for 30s then blotted using force 6 for 12 seconds. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 10 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 2964 / Average electron dose: 51.0 e/Å2 / Details: movie mode 40 frames |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -2.5 µm / Nominal defocus min: -1.0 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Overall B value: 101.45 / Target criteria: CC |
|---|---|
| Output model |  PDB-7lby: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)