[English] 日本語
 Yorodumi
Yorodumi- PDB-7w49: Crystal structure of the gastric proton pump complexed with soraprazan -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7w49 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the gastric proton pump complexed with soraprazan | ||||||
 Components Components | (Potassium-transporting ATPase ...) x 2 | ||||||
 Keywords Keywords | HYDROLASE / Cation pump / P-type ATPase / gastric / proton pump / membrane protein / P-CAB | ||||||
| Function / homology |  Function and homology information Function and homology informationH+/K+-exchanging ATPase / potassium:proton exchanging ATPase complex / P-type potassium:proton transporter activity / Ion transport by P-type ATPases / P-type sodium:potassium-exchanging transporter activity / sodium:potassium-exchanging ATPase complex / sodium ion export across plasma membrane / intracellular sodium ion homeostasis / potassium ion import across plasma membrane / intracellular potassium ion homeostasis ...H+/K+-exchanging ATPase / potassium:proton exchanging ATPase complex / P-type potassium:proton transporter activity / Ion transport by P-type ATPases / P-type sodium:potassium-exchanging transporter activity / sodium:potassium-exchanging ATPase complex / sodium ion export across plasma membrane / intracellular sodium ion homeostasis / potassium ion import across plasma membrane / intracellular potassium ion homeostasis / potassium ion binding / ATPase activator activity / potassium ion transmembrane transport / proton transmembrane transport / cell adhesion / apical plasma membrane / magnesium ion binding / ATP hydrolysis activity / ATP binding / plasma membrane Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.1 Å MOLECULAR REPLACEMENT / Resolution: 3.1 Å | ||||||
 Authors Authors | Abe, K. / Tanaka, S. | ||||||
| Funding support |  Japan, 1items Japan, 1items
| ||||||
 Citation Citation |  Journal: J Med Chem / Year: 2022 Journal: J Med Chem / Year: 2022Title: Structural Basis for Binding of Potassium-Competitive Acid Blockers to the Gastric Proton Pump. Authors: Saki Tanaka / Mikio Morita / Tatsuya Yamagishi / Hridya Valia Madapally / Kenichi Hayashida / Himanshu Khandelia / Christoph Gerle / Hideki Shigematsu / Atsunori Oshima / Kazuhiro Abe /   Abstract: As specific inhibitors of the gastric proton pump, responsible for gastric acidification, K-competitive acid blockers (P-CABs) have recently been utilized in the clinical treatment of gastric acid- ...As specific inhibitors of the gastric proton pump, responsible for gastric acidification, K-competitive acid blockers (P-CABs) have recently been utilized in the clinical treatment of gastric acid-related diseases in Asia. However, as these compounds have been developed based on phenotypic screening, their detailed binding poses are unknown. We show crystal and cryo-EM structures of the gastric proton pump in complex with four different P-CABs, tegoprazan, soraprazan, PF-03716556 and revaprazan, at resolutions reaching 2.8 Å. The structures describe molecular details of their interactions and are supported by functional analyses of mutations and molecular dynamics simulations. We reveal that revaprazan has a novel binding mode in which its tetrahydroisoquinoline moiety binds deep in the cation transport conduit. The mechanism of action of these P-CABs can now be evaluated at the molecular level, which will facilitate the rational development and improvement of currently available P-CABs to provide better treatment of acid-related gastrointestinal diseases. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7w49.cif.gz 7w49.cif.gz | 602.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7w49.ent.gz pdb7w49.ent.gz | 411.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7w49.json.gz 7w49.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7w49_validation.pdf.gz 7w49_validation.pdf.gz | 837.7 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7w49_full_validation.pdf.gz 7w49_full_validation.pdf.gz | 870.7 KB | Display | |
| Data in XML |  7w49_validation.xml.gz 7w49_validation.xml.gz | 47.4 KB | Display | |
| Data in CIF |  7w49_validation.cif.gz 7w49_validation.cif.gz | 63.4 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/w4/7w49 https://data.pdbj.org/pub/pdb/validation_reports/w4/7w49 ftp://data.pdbj.org/pub/pdb/validation_reports/w4/7w49 ftp://data.pdbj.org/pub/pdb/validation_reports/w4/7w49 | HTTPS FTP |
-Related structure data
| Related structure data |  7w47C  7w48C  7w4aC  5ylvS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
|
- Components
Components
-Potassium-transporting ATPase ... , 2 types, 2 molecules AB
| #1: Protein | Mass: 114456.734 Da / Num. of mol.: 1 / Mutation: R221C, S594C, G1006S Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / References: UniProt: P19156, H+/K+-exchanging ATPase Homo sapiens (human) / References: UniProt: P19156, H+/K+-exchanging ATPase |
|---|---|
| #2: Protein | Mass: 33113.844 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / References: UniProt: P18434 Homo sapiens (human) / References: UniProt: P18434 |
-Sugars , 1 types, 3 molecules 
| #6: Sugar |
|---|
-Non-polymers , 3 types, 4 molecules 

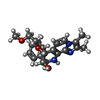


| #3: Chemical | ChemComp-MG / | ||
|---|---|---|---|
| #4: Chemical | | #5: Chemical | ChemComp-8CE / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.04 Å3/Da / Density % sol: 69.56 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop Details: 10% glycerol, 0.2 M RbCl, 20% PEG 2000MME, 3% tert-BuOH, 5 mM beta-MeSH |
-Data collection
| Diffraction | Mean temperature: 77 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SPring-8 SPring-8  / Beamline: BL41XU / Wavelength: 1 Å / Beamline: BL41XU / Wavelength: 1 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Oct 21, 2020 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 3.1→48.53 Å / Num. obs: 44513 / % possible obs: 71.01 % / Redundancy: 2 % / Biso Wilson estimate: 90.91 Å2 / CC1/2: 1 / Net I/σ(I): 16.26 |
| Reflection shell | Resolution: 3.1→3.21 Å / Num. unique obs: 1002 / CC1/2: 0.665 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5ylv Resolution: 3.1→48.53 Å / SU ML: 0.4393 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 39.871 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 87.08 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.1→48.53 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 37.5619998793 Å / Origin y: -32.111178129 Å / Origin z: -42.3233203161 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: all |
 Movie
Movie Controller
Controller























 PDBj
PDBj






