+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6t90 | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | OCT4-SOX2-bound nucleosome - SHL-6 | ||||||||||||||||||||||||||||||||||||||||||
 Components Components |
| ||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | TRANSCRIPTION / nucleosome / OCT4 / SOX2 / transcription factor | ||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationglial cell fate commitment / regulation of myofibroblast cell apoptotic process / Formation of the posterior neural plate / cell fate commitment involved in formation of primary germ layer / cardiac cell fate determination / POU5F1 (OCT4), SOX2, NANOG repress genes related to differentiation / Formation of the anterior neural plate / endodermal-mesodermal cell signaling / regulation of asymmetric cell division / response to oxygen-glucose deprivation ...glial cell fate commitment / regulation of myofibroblast cell apoptotic process / Formation of the posterior neural plate / cell fate commitment involved in formation of primary germ layer / cardiac cell fate determination / POU5F1 (OCT4), SOX2, NANOG repress genes related to differentiation / Formation of the anterior neural plate / endodermal-mesodermal cell signaling / regulation of asymmetric cell division / response to oxygen-glucose deprivation / endodermal cell fate specification / adenohypophysis development / heart induction / negative regulation of cell cycle G1/S phase transition / POU5F1 (OCT4), SOX2, NANOG activate genes related to proliferation / Specification of primordial germ cells / Specification of the neural plate border / pituitary gland development / positive regulation of cell-cell adhesion / Regulation of MITF-M-dependent genes involved in extracellular matrix, focal adhesion and epithelial-to-mesenchymal transition / Transcriptional Regulation by MECP2 / Transcriptional regulation of pluripotent stem cells / neuronal stem cell population maintenance / Germ layer formation at gastrulation / eye development / tissue regeneration / response to growth factor / miRNA binding / negative regulation of neuron differentiation / forebrain development / inner ear development / somatic stem cell population maintenance / blastocyst development / anatomical structure morphogenesis / negative regulation of tumor necrosis factor-mediated signaling pathway / BMP signaling pathway / negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / CENP-A containing nucleosome / Packaging Of Telomere Ends / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Transcriptional and post-translational regulation of MITF-M expression and activity / Deposition of new CENPA-containing nucleosomes at the centromere / telomere organization / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / Interleukin-7 signaling / negative regulation of miRNA transcription / RNA Polymerase I Promoter Opening / epigenetic regulation of gene expression / Inhibition of DNA recombination at telomere / Assembly of the ORC complex at the origin of replication / Meiotic synapsis / bioluminescence / SUMOylation of chromatin organization proteins / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression / HCMV Late Events / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / Regulation of endogenous retroelements by KRAB-ZFP proteins / innate immune response in mucosa / Defective pyroptosis / generation of precursor metabolites and energy / HDACs deacetylate histones / positive regulation of cell differentiation / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / Deactivation of the beta-catenin transactivating complex / RNA Polymerase I Promoter Escape / Nonhomologous End-Joining (NHEJ) / lipopolysaccharide binding / Transcriptional regulation by small RNAs / negative regulation of canonical Wnt signaling pathway / Formation of the beta-catenin:TCF transactivating complex / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / HDMs demethylate histones / brain development / G2/M DNA damage checkpoint / NoRC negatively regulates rRNA expression / response to wounding / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / DNA Damage/Telomere Stress Induced Senescence / DNA-binding transcription repressor activity, RNA polymerase II-specific / Pre-NOTCH Transcription and Translation / Meiotic recombination / Activation of anterior HOX genes in hindbrain development during early embryogenesis / Metalloprotease DUBs / RMTs methylate histone arginines / Transcriptional regulation of granulopoiesis / neuron differentiation / HCMV Early Events / sequence-specific double-stranded DNA binding Similarity search - Function | ||||||||||||||||||||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) synthetic construct (others) | ||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.05 Å | ||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Michael, A.K. / Kempf, G. / Cavadini, S. / Bunker, R.D. / Thoma, N.H. | ||||||||||||||||||||||||||||||||||||||||||
| Funding support |  Switzerland, 1items Switzerland, 1items
| ||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Science / Year: 2020 Journal: Science / Year: 2020Title: Mechanisms of OCT4-SOX2 motif readout on nucleosomes. Authors: Alicia K Michael / Ralph S Grand / Luke Isbel / Simone Cavadini / Zuzanna Kozicka / Georg Kempf / Richard D Bunker / Andreas D Schenk / Alexandra Graff-Meyer / Ganesh R Pathare / Joscha ...Authors: Alicia K Michael / Ralph S Grand / Luke Isbel / Simone Cavadini / Zuzanna Kozicka / Georg Kempf / Richard D Bunker / Andreas D Schenk / Alexandra Graff-Meyer / Ganesh R Pathare / Joscha Weiss / Syota Matsumoto / Lukas Burger / Dirk Schübeler / Nicolas H Thomä /  Abstract: Transcription factors (TFs) regulate gene expression through chromatin where nucleosomes restrict DNA access. To study how TFs bind nucleosome-occupied motifs, we focused on the reprogramming factors ...Transcription factors (TFs) regulate gene expression through chromatin where nucleosomes restrict DNA access. To study how TFs bind nucleosome-occupied motifs, we focused on the reprogramming factors OCT4 and SOX2 in mouse embryonic stem cells. We determined TF engagement throughout a nucleosome at base-pair resolution in vitro, enabling structure determination by cryo-electron microscopy at two preferred positions. Depending on motif location, OCT4 and SOX2 differentially distort nucleosomal DNA. At one position, OCT4-SOX2 removes DNA from histone H2A and histone H3; however, at an inverted motif, the TFs only induce local DNA distortions. OCT4 uses one of its two DNA-binding domains to engage DNA in both structures, reading out a partial motif. These findings explain site-specific nucleosome engagement by the pluripotency factors OCT4 and SOX2, and they reveal how TFs distort nucleosomes to access chromatinized motifs. | ||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6t90.cif.gz 6t90.cif.gz | 362.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6t90.ent.gz pdb6t90.ent.gz | 264.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6t90.json.gz 6t90.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6t90_validation.pdf.gz 6t90_validation.pdf.gz | 909.2 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6t90_full_validation.pdf.gz 6t90_full_validation.pdf.gz | 916.6 KB | Display | |
| Data in XML |  6t90_validation.xml.gz 6t90_validation.xml.gz | 33 KB | Display | |
| Data in CIF |  6t90_validation.cif.gz 6t90_validation.cif.gz | 53.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/t9/6t90 https://data.pdbj.org/pub/pdb/validation_reports/t9/6t90 ftp://data.pdbj.org/pub/pdb/validation_reports/t9/6t90 ftp://data.pdbj.org/pub/pdb/validation_reports/t9/6t90 | HTTPS FTP |
-Related structure data
| Related structure data |  10406MC  6t93C  6yovC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 7 types, 10 molecules ABFCGDHEKL
| #1: Protein | Mass: 15719.445 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) Homo sapiens (human)Gene: HIST1H3A, H3FA, HIST1H3B, H3FL, HIST1H3C, H3FC, HIST1H3D, H3FB, HIST1H3E, H3FD, HIST1H3F, H3FI, HIST1H3G, H3FH, HIST1H3H, H3FK, HIST1H3I, H3FF, HIST1H3J, H3FJ Production host:  References: UniProt: P68431 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| #2: Protein | Mass: 11676.703 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) Homo sapiens (human)Gene: HIST1H4A, H4/A, H4FA, HIST1H4B, H4/I, H4FI, HIST1H4C, H4/G, H4FG, HIST1H4D, H4/B, H4FB, HIST1H4E, H4/J, H4FJ, HIST1H4F, H4/C, H4FC, HIST1H4H, H4/H, H4FH, HIST1H4I, H4/M, H4FM, HIST1H4J, H4/E, ...Gene: HIST1H4A, H4/A, H4FA, HIST1H4B, H4/I, H4FI, HIST1H4C, H4/G, H4FG, HIST1H4D, H4/B, H4FB, HIST1H4E, H4/J, H4FJ, HIST1H4F, H4/C, H4FC, HIST1H4H, H4/H, H4FH, HIST1H4I, H4/M, H4FM, HIST1H4J, H4/E, H4FE, HIST1H4K, H4/D, H4FD, HIST1H4L, H4/K, H4FK, HIST2H4A, H4/N, H4F2, H4FN, HIST2H4, HIST2H4B, H4/O, H4FO, HIST4H4 Production host:  References: UniProt: P62805 #3: Protein | Mass: 14447.825 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: HIST1H2AB, H2AFM, HIST1H2AE, H2AFA Homo sapiens (human) / Gene: HIST1H2AB, H2AFM, HIST1H2AE, H2AFAProduction host:  References: UniProt: P04908 #4: Protein | Mass: 14088.336 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: HIST1H2BJ, H2BFR Homo sapiens (human) / Gene: HIST1H2BJ, H2BFRProduction host:  References: UniProt: P06899 #5: Protein | | Mass: 15491.173 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) Homo sapiens (human)Gene: H3C1, H3FA, HIST1H3A, H3C2, H3FL, HIST1H3B, H3C3, H3FC HIST1H3C, H3C4, H3FB, HIST1H3D, H3C6, H3FD, HIST1H3E, H3C7, H3FI, HIST1H3F, H3C8, H3FH, HIST1H3G, H3C10, H3FK, HIST1H3H, H3C11, H3FF, ...Gene: H3C1, H3FA, HIST1H3A, H3C2, H3FL, HIST1H3B, H3C3, H3FC HIST1H3C, H3C4, H3FB, HIST1H3D, H3C6, H3FD, HIST1H3E, H3C7, H3FI, HIST1H3F, H3C8, H3FH, HIST1H3G, H3C10, H3FK, HIST1H3H, H3C11, H3FF, HIST1H3I, H3C12, H3FJ, HIST1H3J Production host:  References: UniProt: P68431 #8: Protein | | Mass: 70521.289 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) Homo sapiens (human)Gene: GFP, POU5F1, OCT3, OCT4, OTF3 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: P42212, UniProt: Q01860 Trichoplusia ni (cabbage looper) / References: UniProt: P42212, UniProt: Q01860#9: Protein | | Mass: 12718.679 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SOX2 / Production host: Homo sapiens (human) / Gene: SOX2 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: P48431 Trichoplusia ni (cabbage looper) / References: UniProt: P48431 |
-DNA chain , 2 types, 2 molecules IJ
| #6: DNA chain | Mass: 46961.957 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
|---|---|
| #7: DNA chain | Mass: 45634.125 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
-Non-polymers , 1 types, 9 molecules 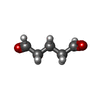
| #10: Chemical | ChemComp-PTD / |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | ||||||||||||||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.4 | ||||||||||||||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||||||||||||||
| Specimen support | Grid material: COPPER / Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 45 e/Å2 / Film or detector model: GATAN K2 QUANTUM (4k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.17rc2_3619: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.05 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 94282 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj













































