+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6rki | ||||||
|---|---|---|---|---|---|---|---|
| Title | Fragment AZ-023 binding at the p53pT387/14-3-3 sigma interface | ||||||
 Components Components |
| ||||||
 Keywords Keywords | PEPTIDE BINDING PROTEIN / protein protein interaction / fragment soaking / stabilization | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of helicase activity / Loss of function of TP53 in cancer due to loss of tetramerization ability / Regulation of TP53 Expression / signal transduction by p53 class mediator / negative regulation of G1 to G0 transition / negative regulation of glucose catabolic process to lactate via pyruvate / Transcriptional activation of cell cycle inhibitor p21 / regulation of intrinsic apoptotic signaling pathway by p53 class mediator / negative regulation of pentose-phosphate shunt / ATP-dependent DNA/DNA annealing activity ...negative regulation of helicase activity / Loss of function of TP53 in cancer due to loss of tetramerization ability / Regulation of TP53 Expression / signal transduction by p53 class mediator / negative regulation of G1 to G0 transition / negative regulation of glucose catabolic process to lactate via pyruvate / Transcriptional activation of cell cycle inhibitor p21 / regulation of intrinsic apoptotic signaling pathway by p53 class mediator / negative regulation of pentose-phosphate shunt / ATP-dependent DNA/DNA annealing activity / Activation of NOXA and translocation to mitochondria / regulation of cell cycle G2/M phase transition / oligodendrocyte apoptotic process / negative regulation of miRNA processing / intrinsic apoptotic signaling pathway in response to hypoxia / positive regulation of thymocyte apoptotic process / oxidative stress-induced premature senescence / regulation of tissue remodeling / positive regulation of mitochondrial membrane permeability / regulation of fibroblast apoptotic process / mRNA transcription / bone marrow development / circadian behavior / positive regulation of programmed necrotic cell death / T cell proliferation involved in immune response / regulation of mitochondrial membrane permeability involved in apoptotic process / histone deacetylase regulator activity / germ cell nucleus / RUNX3 regulates CDKN1A transcription / homolactic fermentation / TP53 Regulates Transcription of Death Receptors and Ligands / TP53 regulates transcription of additional cell cycle genes whose exact role in the p53 pathway remain uncertain / Activation of PUMA and translocation to mitochondria / Regulation of TP53 Activity through Association with Co-factors / regulation of DNA damage response, signal transduction by p53 class mediator / negative regulation of glial cell proliferation / negative regulation of neuroblast proliferation / mitochondrial DNA repair / T cell lineage commitment / Formation of Senescence-Associated Heterochromatin Foci (SAHF) / thymocyte apoptotic process / ER overload response / TP53 Regulates Transcription of Caspase Activators and Caspases / cardiac septum morphogenesis / entrainment of circadian clock by photoperiod / B cell lineage commitment / regulation of epidermal cell division / protein kinase C inhibitor activity / positive regulation of epidermal cell differentiation / keratinocyte development / keratinization / negative regulation of mitophagy / negative regulation of DNA replication / Zygotic genome activation (ZGA) / regulation of cell-cell adhesion / Association of TriC/CCT with target proteins during biosynthesis / TP53 Regulates Transcription of Genes Involved in Cytochrome C Release / PI5P Regulates TP53 Acetylation / necroptotic process / negative regulation of telomere maintenance via telomerase / positive regulation of release of cytochrome c from mitochondria / SUMOylation of transcription factors / TP53 regulates transcription of several additional cell death genes whose specific roles in p53-dependent apoptosis remain uncertain / TFIID-class transcription factor complex binding / cellular response to actinomycin D / intrinsic apoptotic signaling pathway by p53 class mediator / negative regulation of reactive oxygen species metabolic process / rRNA transcription / Transcriptional Regulation by VENTX / establishment of skin barrier / Regulation of localization of FOXO transcription factors / cellular response to UV-C / keratinocyte proliferation / viral process / general transcription initiation factor binding / replicative senescence / intrinsic apoptotic signaling pathway in response to endoplasmic reticulum stress / positive regulation of RNA polymerase II transcription preinitiation complex assembly / neuroblast proliferation / Activation of BAD and translocation to mitochondria / intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / Pyroptosis / phosphoserine residue binding / positive regulation of execution phase of apoptosis / negative regulation of keratinocyte proliferation / embryonic organ development / hematopoietic stem cell differentiation / chromosome organization / cAMP/PKA signal transduction / type II interferon-mediated signaling pathway / negative regulation of protein localization to plasma membrane / response to X-ray / TP53 Regulates Transcription of Genes Involved in G1 Cell Cycle Arrest / somitogenesis / hematopoietic progenitor cell differentiation / SARS-CoV-2 targets host intracellular signalling and regulatory pathways / positive regulation of cardiac muscle cell apoptotic process / core promoter sequence-specific DNA binding / negative regulation of protein kinase activity / glial cell proliferation Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.88 Å MOLECULAR REPLACEMENT / Resolution: 1.88 Å | ||||||
 Authors Authors | Genet, S. / Wolter, M. / Guillory, X. / Somsen, B. / Leysen, S. / Castaldi, P. / Ottmann, C. | ||||||
| Funding support |  Netherlands, 1items Netherlands, 1items
| ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2020 Journal: J.Med.Chem. / Year: 2020Title: Fragment-based Differential Targeting of PPI Stabilizer Interfaces. Authors: Guillory, X. / Wolter, M. / Leysen, S. / Neves, J.F. / Kuusk, A. / Genet, S. / Somsen, B. / Morrow, J.K. / Rivers, E. / van Beek, L. / Patel, J. / Goodnow, R. / Schoenherr, H. / Fuller, N. / ...Authors: Guillory, X. / Wolter, M. / Leysen, S. / Neves, J.F. / Kuusk, A. / Genet, S. / Somsen, B. / Morrow, J.K. / Rivers, E. / van Beek, L. / Patel, J. / Goodnow, R. / Schoenherr, H. / Fuller, N. / Cao, Q. / Doveston, R.G. / Brunsveld, L. / Arkin, M.R. / Castaldi, P. / Boyd, H. / Landrieu, I. / Chen, H. / Ottmann, C. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6rki.cif.gz 6rki.cif.gz | 75.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6rki.ent.gz pdb6rki.ent.gz | 52.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6rki.json.gz 6rki.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rk/6rki https://data.pdbj.org/pub/pdb/validation_reports/rk/6rki ftp://data.pdbj.org/pub/pdb/validation_reports/rk/6rki ftp://data.pdbj.org/pub/pdb/validation_reports/rk/6rki | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6r5lC  6rhcC  6rjlC  6rjqC  6rjzC  6rk8C  6rkkC  6rkmC  6rl3C  6rl4C  6rl6C  6rm5C  6rm7C  6rp6C  6rwhC  6rwiC  6rwsC  6rwuC  6rx2C  6s39C  6s3cC  6s40C  6s9qC  6sinC  6sioC  6sipC  6siqC  6slvC  6slwC  6slxC  5mocS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein / Protein/peptide , 2 types, 2 molecules AP
| #1: Protein | Mass: 28210.518 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: Residues -4 to 0 are expression tag / Source: (gene. exp.)  Homo sapiens (human) / Gene: SFN, HME1 / Production host: Homo sapiens (human) / Gene: SFN, HME1 / Production host:  |
|---|---|
| #2: Protein/peptide | Mass: 1449.520 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.)  Homo sapiens (human) / References: UniProt: P04637 Homo sapiens (human) / References: UniProt: P04637 |
-Non-polymers , 4 types, 383 molecules 

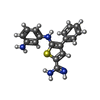




| #3: Chemical | | #4: Chemical | ChemComp-CL / | #5: Chemical | ChemComp-K6T / | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.59 Å3/Da / Density % sol: 52.44 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 0.1M Hepes, pH7.5, 27%PEG, 5% Glycerol, 0.2M Calcium Chloride, 2mM DTT. |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source: SEALED TUBE / Type: RIGAKU MICROMAX-003 / Wavelength: 1.54187 Å |
| Detector | Type: DECTRIS PILATUS 200K / Detector: PIXEL / Date: Aug 10, 2017 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.54187 Å / Relative weight: 1 |
| Reflection | Resolution: 1.88→26.62 Å / Num. all: 144706 / Num. obs: 23804 / % possible obs: 99.8 % / Redundancy: 6.1 % / CC1/2: 0.998 / Rrim(I) all: 0.083 / Net I/σ(I): 15.5 |
| Reflection shell | Resolution: 1.88→1.93 Å / Redundancy: 5.7 % / Mean I/σ(I) obs: 6.3 / Num. unique all: 8396 / Num. unique obs: 1454 / CC1/2: 0.969 / Rrim(I) all: 0.228 / % possible all: 98.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5MOC Resolution: 1.88→26.62 Å / Cor.coef. Fo:Fc: 0.956 / Cor.coef. Fo:Fc free: 0.912 / SU B: 2.455 / SU ML: 0.075 / Cross valid method: FREE R-VALUE / ESU R: 0.124 / ESU R Free: 0.128 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 12.645 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.88→26.62 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller





















 PDBj
PDBj




















