[English] 日本語
 Yorodumi
Yorodumi- PDB-5wbr: Structure of human Ketohexokinase complexed with hits from fragme... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5wbr | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of human Ketohexokinase complexed with hits from fragment screening | ||||||
 Components Components | Ketohexokinase | ||||||
 Keywords Keywords | TRANSFERASE / Ketohexokinase / Fragment-based drug discovery / SBDD | ||||||
| Function / homology |  Function and homology information Function and homology informationEssential fructosuria / ketohexokinase / ketohexokinase activity / fructose binding / Fructose catabolism / regulation of glycogen metabolic process / response to sucrose / response to fructose / fructose metabolic process / response to zinc ion ...Essential fructosuria / ketohexokinase / ketohexokinase activity / fructose binding / Fructose catabolism / regulation of glycogen metabolic process / response to sucrose / response to fructose / fructose metabolic process / response to zinc ion / response to glucose / response to insulin / protein homodimerization activity / extracellular exosome / ATP binding / identical protein binding / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  FOURIER SYNTHESIS / Resolution: 2.58 Å FOURIER SYNTHESIS / Resolution: 2.58 Å | ||||||
 Authors Authors | Pandit, J. | ||||||
 Citation Citation |  Journal: J. Med. Chem. / Year: 2017 Journal: J. Med. Chem. / Year: 2017Title: Discovery of Fragment-Derived Small Molecules for in Vivo Inhibition of Ketohexokinase (KHK). Authors: Huard, K. / Ahn, K. / Amor, P. / Beebe, D.A. / Borzilleri, K.A. / Chrunyk, B.A. / Coffey, S.B. / Cong, Y. / Conn, E.L. / Culp, J.S. / Dowling, M.S. / Gorgoglione, M.F. / Gutierrez, J.A. / ...Authors: Huard, K. / Ahn, K. / Amor, P. / Beebe, D.A. / Borzilleri, K.A. / Chrunyk, B.A. / Coffey, S.B. / Cong, Y. / Conn, E.L. / Culp, J.S. / Dowling, M.S. / Gorgoglione, M.F. / Gutierrez, J.A. / Knafels, J.D. / Lachapelle, E.A. / Pandit, J. / Parris, K.D. / Perez, S. / Pfefferkorn, J.A. / Price, D.A. / Raymer, B. / Ross, T.T. / Shavnya, A. / Smith, A.C. / Subashi, T.A. / Tesz, G.J. / Thuma, B.A. / Tu, M. / Weaver, J.D. / Weng, Y. / Withka, J.M. / Xing, G. / Magee, T.V. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5wbr.cif.gz 5wbr.cif.gz | 133.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5wbr.ent.gz pdb5wbr.ent.gz | 102 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5wbr.json.gz 5wbr.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wb/5wbr https://data.pdbj.org/pub/pdb/validation_reports/wb/5wbr ftp://data.pdbj.org/pub/pdb/validation_reports/wb/5wbr ftp://data.pdbj.org/pub/pdb/validation_reports/wb/5wbr | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5wbmC  5wboC  5wbpC  5wbqC  5wbzC  3nbvS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 34076.586 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: KHK / Production host: Homo sapiens (human) / Gene: KHK / Production host:  |
|---|
-Non-polymers , 5 types, 46 molecules 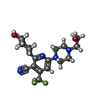








| #2: Chemical | | #3: Chemical | ChemComp-SO4 / #4: Chemical | ChemComp-CIT / | #5: Chemical | ChemComp-GOL / | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.62 Å3/Da / Density % sol: 65.98 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 4.5 Details: 17% PEG 8k, 0.1M Na-Citrate, 0.2M Ammonium sulfate, pH 4.5 Temp details: Room temperature |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 17-ID / Wavelength: 1 Å / Beamline: 17-ID / Wavelength: 1 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: May 16, 2013 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.58→38.255 Å / Num. all: 31956 / Num. obs: 31956 / % possible obs: 99.8 % / Redundancy: 6.6 % / Biso Wilson estimate: 88.08 Å2 / Rpim(I) all: 0.045 / Rrim(I) all: 0.118 / Rsym value: 0.108 / Net I/av σ(I): 7 / Net I/σ(I): 17.4 / Num. measured all: 210546 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: 3NBV Resolution: 2.58→38.26 Å / Cor.coef. Fo:Fc: 0.9447 / Cor.coef. Fo:Fc free: 0.9257 / SU R Cruickshank DPI: 0.324 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.315 / SU Rfree Blow DPI: 0.236 / SU Rfree Cruickshank DPI: 0.24
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 153.96 Å2 / Biso mean: 76.64 Å2 / Biso min: 43.39 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.37 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.58→38.26 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.58→2.67 Å / Rfactor Rfree error: 0 / Total num. of bins used: 16
|
 Movie
Movie Controller
Controller






















 PDBj
PDBj



