[English] 日本語
 Yorodumi
Yorodumi- PDB-5jgq: X-ray structure of neuropilin-1 b1 domain complexed with Arg-7 ligand. -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5jgq | ||||||
|---|---|---|---|---|---|---|---|
| Title | X-ray structure of neuropilin-1 b1 domain complexed with Arg-7 ligand. | ||||||
 Components Components | Neuropilin-1 | ||||||
 Keywords Keywords | SIGNALING PROTEIN / Neuropilin-1 / Angiogenesis | ||||||
| Function / homology |  Function and homology information Function and homology informationendothelial tip cell fate specification / basal dendrite development / otic placode development / protein localization to early endosome / basal dendrite arborization / dichotomous subdivision of terminal units involved in salivary gland branching / retina vasculature morphogenesis in camera-type eye / vestibulocochlear nerve structural organization / dorsal root ganglion morphogenesis / ventral trunk neural crest cell migration ...endothelial tip cell fate specification / basal dendrite development / otic placode development / protein localization to early endosome / basal dendrite arborization / dichotomous subdivision of terminal units involved in salivary gland branching / retina vasculature morphogenesis in camera-type eye / vestibulocochlear nerve structural organization / dorsal root ganglion morphogenesis / ventral trunk neural crest cell migration / sympathetic neuron projection guidance / facioacoustic ganglion development / trigeminal ganglion development / trigeminal nerve structural organization / sensory neuron axon guidance / postsynapse organization / facial nerve structural organization / branchiomotor neuron axon guidance / gonadotrophin-releasing hormone neuronal migration to the hypothalamus / negative regulation of axon extension involved in axon guidance / axon extension involved in axon guidance / VEGF-activated neuropilin signaling pathway / renal artery morphogenesis / neurofilament / sympathetic neuron projection extension / regulation of vascular endothelial growth factor receptor signaling pathway / Neurophilin interactions with VEGF and VEGFR / vascular endothelial growth factor binding / angiogenesis involved in coronary vascular morphogenesis / neural crest cell migration involved in autonomic nervous system development / motor neuron migration / sympathetic ganglion development / positive regulation of axon extension involved in axon guidance / axonogenesis involved in innervation / vascular endothelial growth factor receptor activity / CHL1 interactions / endothelial cell chemotaxis / regulation of vesicle-mediated transport / semaphorin receptor complex / neuropilin signaling pathway / SEMA3A-Plexin repulsion signaling by inhibiting Integrin adhesion / Signaling by ROBO receptors / substrate-dependent cell migration, cell extension / commissural neuron axon guidance / hepatocyte growth factor receptor signaling pathway / coronary artery morphogenesis / semaphorin receptor activity / CRMPs in Sema3A signaling / outflow tract septum morphogenesis / motor neuron axon guidance / axonal fasciculation / cell migration involved in sprouting angiogenesis / sprouting angiogenesis / retinal ganglion cell axon guidance / regulation of Cdc42 protein signal transduction / positive regulation of cell migration involved in sprouting angiogenesis / neural crest cell migration / artery morphogenesis / positive regulation of filopodium assembly / positive regulation of smooth muscle cell migration / cellular response to hepatocyte growth factor stimulus / growth factor binding / branching involved in blood vessel morphogenesis / positive chemotaxis / cytokine binding / sorting endosome / platelet-derived growth factor receptor signaling pathway / semaphorin-plexin signaling pathway / Sema3A PAK dependent Axon repulsion / positive regulation of phosphorylation / cellular response to vascular endothelial growth factor stimulus / positive regulation of focal adhesion assembly / vascular endothelial growth factor receptor signaling pathway / vasculogenesis / coreceptor activity / positive regulation of stress fiber assembly / positive regulation of endothelial cell proliferation / positive regulation of substrate adhesion-dependent cell spreading / positive regulation of endothelial cell migration / axon guidance / GTPase activator activity / animal organ morphogenesis / integrin-mediated signaling pathway / Signal transduction by L1 / negative regulation of extrinsic apoptotic signaling pathway / response to wounding / mitochondrial membrane / neuron migration / positive regulation of angiogenesis / cell-cell signaling / heparin binding / cytoplasmic vesicle / angiogenesis / negative regulation of neuron apoptotic process / postsynaptic membrane / Attachment and Entry / early endosome / positive regulation of ERK1 and ERK2 cascade / receptor complex / neuron projection Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.6 Å MOLECULAR REPLACEMENT / Resolution: 1.6 Å | ||||||
 Authors Authors | Fotinou, C. / Rana, R. / Djordjevic, S. / Yelland, T. | ||||||
 Citation Citation |  Journal: FEBS J. / Year: 2018 Journal: FEBS J. / Year: 2018Title: Architecture and hydration of the arginine-binding site of neuropilin-1. Authors: Mota, F. / Fotinou, C. / Rana, R.R. / Chan, A.W.E. / Yelland, T. / Arooz, M.T. / O'Leary, A.P. / Hutton, J. / Frankel, P. / Zachary, I. / Selwood, D. / Djordjevic, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5jgq.cif.gz 5jgq.cif.gz | 141.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5jgq.ent.gz pdb5jgq.ent.gz | 111.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5jgq.json.gz 5jgq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jg/5jgq https://data.pdbj.org/pub/pdb/validation_reports/jg/5jgq ftp://data.pdbj.org/pub/pdb/validation_reports/jg/5jgq ftp://data.pdbj.org/pub/pdb/validation_reports/jg/5jgq | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5ijrC  5iyyC  5j1xC  5jgiC  5jhkC  1kexS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 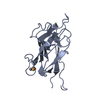
| ||||||||||||||||||
| 2 | 
| ||||||||||||||||||
| Unit cell |
| ||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: _ / Ens-ID: 1 / Beg auth comp-ID: LYS / Beg label comp-ID: LYS / End auth comp-ID: ILE / End label comp-ID: ILE / Refine code: _ / Auth seq-ID: 274 - 426 / Label seq-ID: 5 - 157
|
- Components
Components
| #1: Protein | Mass: 17917.291 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: NRP1, NRP, VEGF165R / Plasmid: pET15B-TEV-NRP1B1 / Production host: Homo sapiens (human) / Gene: NRP1, NRP, VEGF165R / Plasmid: pET15B-TEV-NRP1B1 / Production host:  #2: Chemical | ChemComp-DMS / | #3: Chemical | ChemComp-6JY / | #4: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.09 Å3/Da / Density % sol: 41.04 % |
|---|---|
| Crystal grow | Temperature: 289 K / Method: vapor diffusion, hanging drop / pH: 7.9 / Details: 18%PEG3350+0.2M NH4Cl |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04 / Wavelength: 0.979 Å / Beamline: I04 / Wavelength: 0.979 Å |
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Jul 30, 2015 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.979 Å / Relative weight: 1 |
| Reflection | Resolution: 1.6→40.92 Å / Num. obs: 37578 / % possible obs: 97 % / Redundancy: 3.1 % / CC1/2: 0.998 / Rmerge(I) obs: 0.047 / Net I/σ(I): 10.5 |
| Reflection shell | Resolution: 1.6→1.63 Å / Redundancy: 1.9 % / Rmerge(I) obs: 0.442 / Mean I/σ(I) obs: 2 / % possible all: 75.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1kex Resolution: 1.6→44.78 Å / Cor.coef. Fo:Fc: 0.968 / Cor.coef. Fo:Fc free: 0.958 / SU B: 3.589 / SU ML: 0.063 / Cross valid method: THROUGHOUT / ESU R: 0.09 / ESU R Free: 0.089 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 19.591 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.6→44.78 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller









 PDBj
PDBj