[English] 日本語
 Yorodumi
Yorodumi- EMDB-31178: Structure of Lassa virus polymerase in complex with 3'-vRNA and Z... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-31178 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Lassa virus polymerase in complex with 3'-vRNA and Z mutant (F36A) | |||||||||
 Map data Map data | The EM map of Lassa L in complex with 3'-vRNA and Z mutant (F36A) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Lassa virus / Polymerase / Z protein / replication regulation / VIRAL PROTEIN-RNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative stranded viral RNA replication / cap snatching / viral budding via host ESCRT complex / viral budding from plasma membrane / virion component / host cell / Hydrolases; Acting on ester bonds / host cell cytoplasm / host cell perinuclear region of cytoplasm / hydrolase activity ...negative stranded viral RNA replication / cap snatching / viral budding via host ESCRT complex / viral budding from plasma membrane / virion component / host cell / Hydrolases; Acting on ester bonds / host cell cytoplasm / host cell perinuclear region of cytoplasm / hydrolase activity / RNA-directed RNA polymerase / nucleotide binding / RNA-directed RNA polymerase activity / host cell plasma membrane / RNA binding / zinc ion binding / metal ion binding / membrane Similarity search - Function | |||||||||
| Biological species |  Lassa mammarenavirus Lassa mammarenavirus | |||||||||
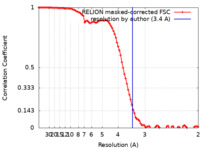
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Xu X / Peng R | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Microbiol / Year: 2021 Journal: Nat Microbiol / Year: 2021Title: Cryo-EM structures of Lassa and Machupo virus polymerases complexed with cognate regulatory Z proteins identify targets for antivirals. Authors: Xin Xu / Ruchao Peng / Qi Peng / Min Wang / Ying Xu / Sheng Liu / Xiaolin Tian / Haiteng Deng / Yimin Tong / Xiaoyou Hu / Jin Zhong / Peiyi Wang / Jianxun Qi / George F Gao / Yi Shi /  Abstract: Zoonotic arenaviruses can lead to life-threating diseases in humans. These viruses encode a large (L) polymerase that transcribes and replicates the viral genome. At the late stage of replication, ...Zoonotic arenaviruses can lead to life-threating diseases in humans. These viruses encode a large (L) polymerase that transcribes and replicates the viral genome. At the late stage of replication, the multifunctional Z protein interacts with the L polymerase to shut down RNA synthesis and initiate virion assembly. However, the mechanism by which the Z protein regulates the activity of L polymerase is unclear. Here, we used cryo-electron microscopy to resolve the structures of both Lassa and Machupo virus L polymerases in complex with their cognate Z proteins, and viral RNA, to 3.1-3.9 Å resolutions. These structures reveal that Z protein binding induces conformational changes in two catalytic motifs of the L polymerase, and restrains their conformational dynamics to inhibit RNA synthesis, which is supported by hydrogen-deuterium exchange mass spectrometry analysis. Importantly, we show, by in vitro polymerase reactions, that Z proteins of Lassa and Machupo viruses can cross-inhibit their L polymerases, albeit with decreased inhibition efficiencies. This cross-reactivity results from a highly conserved determinant motif at the contacting interface, but is affected by other variable auxiliary motifs due to the divergent evolution of Old World and New World arenaviruses. These findings could provide promising targets for developing broad-spectrum antiviral drugs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31178.map.gz emd_31178.map.gz | 4.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31178-v30.xml emd-31178-v30.xml emd-31178.xml emd-31178.xml | 19.4 KB 19.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_31178_fsc.xml emd_31178_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_31178.png emd_31178.png | 55.9 KB | ||
| Filedesc metadata |  emd-31178.cif.gz emd-31178.cif.gz | 7.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31178 http://ftp.pdbj.org/pub/emdb/structures/EMD-31178 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31178 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31178 | HTTPS FTP |
-Validation report
| Summary document |  emd_31178_validation.pdf.gz emd_31178_validation.pdf.gz | 392.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_31178_full_validation.pdf.gz emd_31178_full_validation.pdf.gz | 392.5 KB | Display | |
| Data in XML |  emd_31178_validation.xml.gz emd_31178_validation.xml.gz | 10 KB | Display | |
| Data in CIF |  emd_31178_validation.cif.gz emd_31178_validation.cif.gz | 13.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31178 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31178 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31178 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31178 | HTTPS FTP |
-Related structure data
| Related structure data |  7elaMC  7cklC  7ckmC  7el9C  7elbC  7elcC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_31178.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31178.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The EM map of Lassa L in complex with 3'-vRNA and Z mutant (F36A) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Lassa virus polymerase in complex with 3'-vRNA and Z mutant (F36A).
| Entire | Name: Lassa virus polymerase in complex with 3'-vRNA and Z mutant (F36A). |
|---|---|
| Components |
|
-Supramolecule #1: Lassa virus polymerase in complex with 3'-vRNA and Z mutant (F36A).
| Supramolecule | Name: Lassa virus polymerase in complex with 3'-vRNA and Z mutant (F36A). type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Lassa mammarenavirus Lassa mammarenavirus |
| Molecular weight | Theoretical: 270 KDa |
-Supramolecule #2: Lassa virus Z protein
| Supramolecule | Name: Lassa virus Z protein / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|
-Supramolecule #3: 3-'vRNA promoter
| Supramolecule | Name: 3-'vRNA promoter / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Lassa mammarenavirus / Synthetically produced: Yes Lassa mammarenavirus / Synthetically produced: Yes |
-Supramolecule #4: Lassa virus polymerase
| Supramolecule | Name: Lassa virus polymerase / type: complex / ID: 4 / Parent: 1 / Macromolecule list: #3 |
|---|
-Macromolecule #1: RING finger protein Z
| Macromolecule | Name: RING finger protein Z / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Lassa mammarenavirus Lassa mammarenavirus |
| Molecular weight | Theoretical: 10.665365 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGNKQVKAPE ARNSPRASLI PDATHLGPQF CKSCWAENKG LVECNNHYLC LNCLTLLLGV SSRCPICKMP LPTRLRPSAA PTAPPAEAG DNTRPPPYSP UniProtKB: RING finger protein Z |
-Macromolecule #3: RNA-directed RNA polymerase L
| Macromolecule | Name: RNA-directed RNA polymerase L / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO / EC number: RNA-directed RNA polymerase |
|---|---|
| Source (natural) | Organism:  Lassa mammarenavirus Lassa mammarenavirus |
| Molecular weight | Theoretical: 253.505828 KDa |
| Recombinant expression | Organism:  Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) |
| Sequence | String: MEEDIACVKD LVSKYLANNE RLSRQKLAFL VQTEPRMLLM EGLKLLSLCI EVDSCNANGC EHNSEDKSVE RILHDHGVLT PSLCFVVPD GYKLTGNVLI LLECFVRSSP ANFEQKYVED FKKLEQLKED LKSVDINLIP LIDGRTSFYN EQIPDWVNDK L RDTLFSLL ...String: MEEDIACVKD LVSKYLANNE RLSRQKLAFL VQTEPRMLLM EGLKLLSLCI EVDSCNANGC EHNSEDKSVE RILHDHGVLT PSLCFVVPD GYKLTGNVLI LLECFVRSSP ANFEQKYVED FKKLEQLKED LKSVDINLIP LIDGRTSFYN EQIPDWVNDK L RDTLFSLL KYAQESNSLF EESEYSRLCE SLSMTSGRLS GVESLNALLD NRSNHYEEVI ASCHQGINNK LTAHEVKLQI EE EYQVFRN RLRKGEIEGQ FLKVEKNQLL NEFNNLYADK VAEKDSVEHL THQFKRASPI LRFLYANISK GDNGEGNLII GEC QMQCWR SFLNKVKSMR ILNTRRKLLL IFDALILLAS KHDQVRKKPL RGWLGTCFVS VNDRLVSLES TKKDLKKWVE RRQQ VERSR TMQSFQCPSK NQILNSIFQK TISKATTALR DVGISVDHYK IDMEVICPDG YDLIMDFDVS GVTPTISYQR SEEEA FPYI MGDVDLLKTT DLERLSSLSL ALVNSMKTSS TVKLRQNEFG PARYQVVKCK EAYCQEFSLG ETEFQLIYQK TGECSK CYA INDNRVGEVC SFYADPKRYF PAIFSAEVLQ TTVSTMISWI EDCNELEEQL DKIRSLTKMI LILILAHPSK RSQKLLQ NL RYFIMAYVSD YYHKDLIDKV REELITDVEF LLYRLLRTLM GLVLSEDVKS MMTNRFKFIL NISYMCHFIT KETPDRLT D QIKCFEKFLE PKVKFGHVSI NPADTATEEE LDDMVYNAKK FLSKGGCTSA KGPSYKKPGV SKKYLSLLTS SFNNGSLFK EREVKKEIKD PLITSGCATA LDLASNKSVV VNKYTDGSRV LNYDFNKLTA LAVSQLTEVF SRKGKHLLNK QDYEYKVQQA MSNLVLGSK QHKGDADEAD LDEILLDGGA STYFNQLKET VEKIVDQYRE PVKMGSGSND GDQPSINDLD EIVSNKFYIR L IKGELSNH MVEDFDHDVL PDKFYEEFCD AVYENSKLKE KYFYCGHMSQ CPIGELTKAV STRTYLDHEY FQCFKSILLI MN ANALMGK YTHYKSRNLN FKFDMGKLSD DARISERESN SEALSKALSL TNCTTAMLKN LCFYSQESPQ SYNSVGPDTG RLK FSLSYK EQVGGNRELY IGDLRTKMFT RLIEDYFEAL SSQLSGSCLN NEKEFENAIL SMKLNVSMAH VSYSMDHSKW GPMM CPFLF LTVLQNLIFL SKDLQADIKG RDYLSTLLMW HMHKMVEIPF NVVSAMMKSF IKAQLGLRKK TKQSITEDFF YSNFQ IGVV PSHISSILDM GQGILHNTSD FYALITERFI NYAISCVCGG TIDAYTSSDD QISLFDQTLT ELLHRDPEEF RALMEF HYY MSDQLNKFVS PKSVIGRFVA EFKSRFFVWG DEVPLLTKFV AAALHNIKCK EPHQLAETID TIVDQSVANG VPVHLCN LI QIRTLSLLQY ARYPIDPFLL NCETDVRDWV DGNRSYRIMR QIEGLIPDAC SKIRSMLRRL YNRLKTGQLH EEFTTNYL S SEHLSSLKNL CELLGVEPPS ESDLEYSWLN LAAHHPLRMV LRQKIIYSGA VNLDDEKIPT IVKTIQNKLS STFTRGAQK LLSEAINKSA FQSSIASGFV GLCRTLGSKC VRGPNKENLY IKSIQSLITG TQGIELLTNS IGVQYWRVPL GLRNKSESVV SYFRPLLWD YMCISLSTAI ELGAWVLGDP KTTKALDFFK HNPCDYFPLK PTASKLLEDR VGLNHIIHSL RRLYPSVFEK H ILPFMSDL ASTKMKWSPR IKFLDLCVAL DVNCEALSLV SHIVKWKREE HYIVLSSELR FSHTRTHEPM VEERVVSTSD AV DNFMRQI YFESYVRPFV ATTRTLGSFT WFPHRTSIPE GEGLHRLGPF SSFVEKVIHK GVERPMFKHD LMMGYAWIDF DIE PARFNQ NQLIASGLVD SKFDSLEDFF DAVASLPTGS AKLSQTVRFR IKSQDASFRE SFAIHLDYIG SMNQQAKYLV HDVT AMYSG AVSPCVLSDC WRLVLSGPTF KGKPVWYVDT EVINEFLVDT NQLGHVTPVE VVVDMEKLQF AEYDFMLVGP CAEPV PLVV RRGGLWECEK KLASFTPVIQ DQDLEMFVRE VGDTSSDLLI RALSDMITDR LGLRMQWSGV DIVSTLRAAA PGNAEV LSA VLEVVDNWVE FKGYALCYSK SRGRVMVQSS SGKLRLKGRT CEELTEGGEH VEDIE UniProtKB: RNA-directed RNA polymerase L |
-Macromolecule #2: 3-'vRNA promoter
| Macromolecule | Name: 3-'vRNA promoter / type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  Lassa mammarenavirus Lassa mammarenavirus |
| Molecular weight | Theoretical: 6.069649 KDa |
| Sequence | String: GCCUAGGAUC CACUGUGCG |
-Macromolecule #4: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 4 / Number of copies: 2 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #5: MANGANESE (II) ION
| Macromolecule | Name: MANGANESE (II) ION / type: ligand / ID: 5 / Number of copies: 1 / Formula: MN |
|---|---|
| Molecular weight | Theoretical: 54.938 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: NICKEL/TITANIUM / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY Details: The film was designed with regular 1.2/1.3 micron holey partterns, similar to the Quantifoil 1.2/1.3 holey grids. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 3-18 / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: Correlation coefficient | ||||||
| Output model |  PDB-7ela: |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)