[English] 日本語
 Yorodumi
Yorodumi- EMDB-25522: Cryo-EM structure of the extracellular module of the full-length ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-25522 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
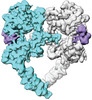
| Title | Cryo-EM structure of the extracellular module of the full-length EGFR bound to EGF "tips-juxtaposed" conformation | |||||||||
 Map data Map data | WT:EGF_jux | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | receptor tyrosine kinases / epidermal growth factor receptor / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of hyaluronan biosynthetic process / negative regulation of secretion / negative regulation of cholesterol efflux / positive regulation of epithelial tube formation / positive regulation of cerebellar granule cell precursor proliferation / positive regulation of protein localization to early endosome / regulation of protein localization to cell surface / cerebellar granule cell precursor proliferation / regulation of calcium ion import / transmembrane receptor protein tyrosine kinase activator activity ...positive regulation of hyaluronan biosynthetic process / negative regulation of secretion / negative regulation of cholesterol efflux / positive regulation of epithelial tube formation / positive regulation of cerebellar granule cell precursor proliferation / positive regulation of protein localization to early endosome / regulation of protein localization to cell surface / cerebellar granule cell precursor proliferation / regulation of calcium ion import / transmembrane receptor protein tyrosine kinase activator activity / Developmental Lineage of Pancreatic Acinar Cells / positive regulation of ubiquitin-dependent protein catabolic process / Differentiation of Keratinocytes in Interfollicular Epidermis in Mammalian Skin / multivesicular body, internal vesicle lumen / negative regulation of cardiocyte differentiation / Shc-EGFR complex / epidermal growth factor receptor binding / positive regulation of protein kinase C signaling / positive regulation of peptidyl-threonine phosphorylation / Inhibition of Signaling by Overexpressed EGFR / EGFR interacts with phospholipase C-gamma / epidermal growth factor receptor activity / regulation of receptor signaling pathway via JAK-STAT / regulation of peptidyl-tyrosine phosphorylation / positive regulation of DNA binding / epidermal growth factor binding / NFE2L2 regulating tumorigenic genes / response to UV-A / PLCG1 events in ERBB2 signaling / ERBB2-EGFR signaling pathway / morphogenesis of an epithelial fold / PTK6 promotes HIF1A stabilization / ERBB2 Activates PTK6 Signaling / digestive tract morphogenesis / branching morphogenesis of an epithelial tube / Signaling by EGFR / intracellular vesicle / eyelid development in camera-type eye / negative regulation of epidermal growth factor receptor signaling pathway / cerebral cortex cell migration / protein insertion into membrane / ERBB2 Regulates Cell Motility / protein tyrosine kinase activator activity / Respiratory syncytial virus (RSV) attachment and entry / positive regulation of receptor internalization / Signaling by ERBB4 / PI3K events in ERBB2 signaling / positive regulation of phosphorylation / positive regulation of peptidyl-serine phosphorylation / mammary gland alveolus development / Estrogen-dependent nuclear events downstream of ESR-membrane signaling / hair follicle development / MAP kinase kinase kinase activity / GAB1 signalosome / positive regulation of G1/S transition of mitotic cell cycle / embryonic placenta development / salivary gland morphogenesis / ERK1 and ERK2 cascade / positive regulation of endothelial cell proliferation / Signaling by ERBB2 / GRB2 events in EGFR signaling / TFAP2 (AP-2) family regulates transcription of growth factors and their receptors / SHC1 events in EGFR signaling / transmembrane receptor protein tyrosine kinase activity / EGFR Transactivation by Gastrin / positive regulation of endothelial cell migration / GRB2 events in ERBB2 signaling / positive regulation of mitotic nuclear division / SHC1 events in ERBB2 signaling / ossification / NOTCH3 Activation and Transmission of Signal to the Nucleus / positive regulation of DNA repair / basal plasma membrane / cellular response to epidermal growth factor stimulus / guanyl-nucleotide exchange factor activity / platelet alpha granule lumen / EGFR downregulation / positive regulation of DNA replication / epithelial cell proliferation / Signal transduction by L1 / positive regulation of epithelial cell proliferation / positive regulation of protein localization to plasma membrane / cellular response to amino acid stimulus / growth factor activity / phosphatidylinositol 3-kinase/protein kinase B signal transduction / cellular response to estradiol stimulus / clathrin-coated endocytic vesicle membrane / Signaling by ERBB2 TMD/JMD mutants / Constitutive Signaling by EGFRvIII / Downregulation of ERBB2 signaling / cell-cell adhesion / receptor protein-tyrosine kinase / negative regulation of protein catabolic process / Signaling by ERBB2 ECD mutants / Signaling by ERBB2 KD Mutants / positive regulation of miRNA transcription / kinase binding / ruffle membrane / positive regulation of protein phosphorylation / epidermal growth factor receptor signaling pathway Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Huang Y / Ognjenovic J | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2021 Journal: Elife / Year: 2021Title: A molecular mechanism for the generation of ligand-dependent differential outputs by the epidermal growth factor receptor. Authors: Yongjian Huang / Jana Ognjenovic / Deepti Karandur / Kate Miller / Alan Merk / Sriram Subramaniam / John Kuriyan /   Abstract: The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that couples the binding of extracellular ligands, such as EGF and transforming growth factor-α (TGF-α), to the initiation ...The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that couples the binding of extracellular ligands, such as EGF and transforming growth factor-α (TGF-α), to the initiation of intracellular signaling pathways. EGFR binds to EGF and TGF-α with similar affinity, but generates different signals from these ligands. To address the mechanistic basis of this phenomenon, we have carried out cryo-EM analyses of human EGFR bound to EGF and TGF-α. We show that the extracellular module adopts an ensemble of dimeric conformations when bound to either EGF or TGF-α. The two extreme states of this ensemble represent distinct ligand-bound quaternary structures in which the membrane-proximal tips of the extracellular module are either juxtaposed or separated. EGF and TGF-α differ in their ability to maintain the conformation with the membrane-proximal tips of the extracellular module separated, and this conformation is stabilized preferentially by an oncogenic EGFR mutation. Close proximity of the transmembrane helices at the junction with the extracellular module has been associated previously with increased EGFR activity. Our results show how EGFR can couple the binding of different ligands to differential modulation of this proximity, thereby suggesting a molecular mechanism for the generation of ligand-sensitive differential outputs in this receptor family. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25522.map.gz emd_25522.map.gz | 52 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25522-v30.xml emd-25522-v30.xml emd-25522.xml emd-25522.xml | 13.1 KB 13.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_25522.png emd_25522.png | 183.2 KB | ||
| Filedesc metadata |  emd-25522.cif.gz emd-25522.cif.gz | 6.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25522 http://ftp.pdbj.org/pub/emdb/structures/EMD-25522 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25522 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25522 | HTTPS FTP |
-Validation report
| Summary document |  emd_25522_validation.pdf.gz emd_25522_validation.pdf.gz | 482.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_25522_full_validation.pdf.gz emd_25522_full_validation.pdf.gz | 482.3 KB | Display | |
| Data in XML |  emd_25522_validation.xml.gz emd_25522_validation.xml.gz | 6.6 KB | Display | |
| Data in CIF |  emd_25522_validation.cif.gz emd_25522_validation.cif.gz | 7.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25522 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25522 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25522 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25522 | HTTPS FTP |
-Related structure data
| Related structure data |  7sydMC  7syeC  7sz0C  7sz1C  7sz5C  7sz7C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25522.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25522.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | WT:EGF_jux | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0794 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : full-length human EGFR:EGF complex
| Entire | Name: full-length human EGFR:EGF complex |
|---|---|
| Components |
|
-Supramolecule #1: full-length human EGFR:EGF complex
| Supramolecule | Name: full-length human EGFR:EGF complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Epidermal growth factor receptor
| Macromolecule | Name: Epidermal growth factor receptor / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: receptor protein-tyrosine kinase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 134.433328 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MRPSGTAGAA LLALLAALCP ASRALEEKKV CQGTSNKLTQ LGTFEDHFLS LQRMFNNCEV VLGNLEITYV QRNYDLSFLK TIQEVAGYV LIALNTVERI PLENLQIIRG NMYYENSYAL AVLSNYDANK TGLKELPMRN LQEILHGAVR FSNNPALCNV E SIQWRDIV ...String: MRPSGTAGAA LLALLAALCP ASRALEEKKV CQGTSNKLTQ LGTFEDHFLS LQRMFNNCEV VLGNLEITYV QRNYDLSFLK TIQEVAGYV LIALNTVERI PLENLQIIRG NMYYENSYAL AVLSNYDANK TGLKELPMRN LQEILHGAVR FSNNPALCNV E SIQWRDIV SSDFLSNMSM DFQNHLGSCQ KCDPSCPNGS CWGAGEENCQ KLTKIICAQQ CSGRCRGKSP SDCCHNQCAA GC TGPRESD CLVCRKFRNE ATCKDTCPPL MLYNPTTYQM DVNPEGKYSF GATCVKKCPR NYVVTDHGSC VRACGADSYE MEE DGVRKC KKCEGPCRKV CNGIGIGEFK DSLSINATNI KHFKNCTSIS GDLHILPVAF RGDSFTHTPP LDPQELDILK TVKE ITGFL LIQAWPENRT DLHAFENLEI IRGRTKQHGQ FSLAVVSLNI TSLGLRSLKE ISDGDVIISG NKNLCYANTI NWKKL FGTS GQKTKIISNR GENSCKATGQ VCHALCSPEG CWGPEPRDCV SCRNVSRGRE CVDKCNLLEG EPREFVENSE CIQCHP ECL PQAMNITCTG RGPDNCIQCA HYIDGPHCVK TCPAGVMGEN NTLVWKYADA GHVCHLCHPN CTYGCTGPGL EGCPTNG PK IPSIATGMVG ALLLLLVVAL GIGLFMRRRH IVRKRTLRRL LQERELVEPL TPSGEAPNQA LLRILKETEF KKIKVLGS G AFGTVYKGLW IPEGEKVKIP VAIKELREAT SPKANKEILD EAYVMASVDN PHVCRLLGIC LTSTVQLITQ LMPFGCLLD YVREHKDNIG SQYLLNWCVQ IAKGMNYLED RRLVHRDLAA RNVLVKTPQH VKITDFGLAK LLGAEEKEYH AEGGKVPIKW MALESILHR IYTHQSDVWS YGVTVWELMT FGSKPYDGIP ASEISSILEK GERLPQPPIC TIDVYMIMVK CWMIDADSRP K FRELIIEF SKMARDPQRY LVIQGDERMH LPSPTDSNFY RALMDEEDMD DVVDADEYLI PQQGFFSSPS TSRTPLLSSL SA TSNNSTV ACIDRNGLQS CPIKEDSFLQ RYSSDPTGAL TEDSIDDTFL PVPEYINQSV PKRPAGSVQN PVYHNQPLNP APS RDPHYQ DPHSTAVGNP EYLNTVQPTC VNSTFDSPAH WAQKGSHQIS LDNPDYQQDF FPKEAKPNGI FKGSTAENAE YLRV APQSS EFIGA UniProtKB: Epidermal growth factor receptor |
-Macromolecule #2: Epidermal growth factor
| Macromolecule | Name: Epidermal growth factor / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 6.229027 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: NSDSECPLSH DGYCLHDGVC MYIEALDKYA CNCVVGYIGE RCQYRDLKWW ELR UniProtKB: Pro-epidermal growth factor |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: PLASMA CLEANING |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 7.5 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















