[English] 日本語
 Yorodumi
Yorodumi- EMDB-23640: Cryo-EM structure of the HCMV pentamer bound by antibodies 1-103,... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23640 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the HCMV pentamer bound by antibodies 1-103, 1-32 and 2-25 | |||||||||
 Map data Map data | Focused refinement calculated in cryoSPARC and sharpened using DeepEMhancer. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | HCMV pentamer / Fab / cytomegalovirus / immunocomplex / VIRAL PROTEIN / VIRAL PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationhost cell Golgi apparatus / entry receptor-mediated virion attachment to host cell / fusion of virus membrane with host plasma membrane / viral envelope / symbiont entry into host cell / host cell plasma membrane / virion membrane Similarity search - Function | |||||||||
| Biological species |   Human betaherpesvirus 5 / Human betaherpesvirus 5 /  Homo sapiens (human) / Homo sapiens (human) /   Human cytomegalovirus Human cytomegalovirus | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.81 Å | |||||||||
 Authors Authors | Wrapp D / McLellan JS | |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2022 Journal: Sci Adv / Year: 2022Title: Structural basis for HCMV Pentamer recognition by neuropilin 2 and neutralizing antibodies. Authors: Daniel Wrapp / Xiaohua Ye / Zhiqiang Ku / Hang Su / Harrison G Jones / Nianshuang Wang / Akaash K Mishra / Daniel C Freed / Fengsheng Li / Aimin Tang / Leike Li / Dabbu Kumar Jaijyan / Hua ...Authors: Daniel Wrapp / Xiaohua Ye / Zhiqiang Ku / Hang Su / Harrison G Jones / Nianshuang Wang / Akaash K Mishra / Daniel C Freed / Fengsheng Li / Aimin Tang / Leike Li / Dabbu Kumar Jaijyan / Hua Zhu / Dai Wang / Tong-Ming Fu / Ningyan Zhang / Zhiqiang An / Jason S McLellan /  Abstract: Human cytomegalovirus (HCMV) encodes multiple surface glycoprotein complexes to infect a variety of cell types. The HCMV Pentamer, composed of gH, gL, UL128, UL130, and UL131A, enhances entry into ...Human cytomegalovirus (HCMV) encodes multiple surface glycoprotein complexes to infect a variety of cell types. The HCMV Pentamer, composed of gH, gL, UL128, UL130, and UL131A, enhances entry into epithelial, endothelial, and myeloid cells by interacting with the cell surface receptor neuropilin 2 (NRP2). Despite the critical nature of this interaction, the molecular determinants that govern NRP2 recognition remain unclear. Here, we describe the cryo-EM structure of NRP2 bound to Pentamer. The high-affinity interaction between these proteins is calcium dependent and differs from the canonical carboxyl-terminal arginine (CendR) binding that NRP2 typically uses. We also determine the structures of four neutralizing human antibodies bound to the HCMV Pentamer to define susceptible epitopes. Two of these antibodies compete with NRP2 binding, but the two most potent antibodies recognize a previously unidentified epitope that does not overlap the NRP2-binding site. Collectively, these findings provide a structural basis for HCMV tropism and antibody-mediated neutralization. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23640.map.gz emd_23640.map.gz | 212.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23640-v30.xml emd-23640-v30.xml emd-23640.xml emd-23640.xml | 32.6 KB 32.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23640_fsc.xml emd_23640_fsc.xml | 15.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_23640.png emd_23640.png | 120.1 KB | ||
| Filedesc metadata |  emd-23640.cif.gz emd-23640.cif.gz | 7.6 KB | ||
| Others |  emd_23640_additional_1.map.gz emd_23640_additional_1.map.gz emd_23640_half_map_1.map.gz emd_23640_half_map_1.map.gz emd_23640_half_map_2.map.gz emd_23640_half_map_2.map.gz | 237.9 MB 285.2 MB 285.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23640 http://ftp.pdbj.org/pub/emdb/structures/EMD-23640 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23640 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23640 | HTTPS FTP |
-Related structure data
| Related structure data |  7m30MC  7kbaC  7kbbC  7lyvC  7lywC  7m1cC  7m22C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_23640.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23640.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Focused refinement calculated in cryoSPARC and sharpened using DeepEMhancer. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.075 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Full map that was later subjected to focused...
| File | emd_23640_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Full map that was later subjected to focused refinement to calculate the primary map. Calculated in cryoSPARC and sharpened using DeepEMhancer. | ||||||||||||
| Projections & Slices |
| ||||||||||||
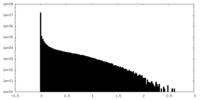
| Density Histograms |
-Half map: Half map A.
| File | emd_23640_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A. | ||||||||||||
| Projections & Slices |
| ||||||||||||
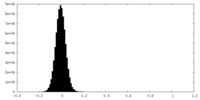
| Density Histograms |
-Half map: Half map B.
| File | emd_23640_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B. | ||||||||||||
| Projections & Slices |
| ||||||||||||
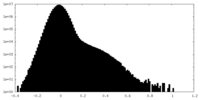
| Density Histograms |
- Sample components
Sample components
+Entire : Quaternary complex of the HCMV pentamer bound by Fabs 1-103, 1-32...
+Supramolecule #1: Quaternary complex of the HCMV pentamer bound by Fabs 1-103, 1-32...
+Supramolecule #2: HCMV pentamer
+Supramolecule #3: 2-25 Fab
+Supramolecule #4: 1-32 Fab
+Supramolecule #5: 1-103 Fab
+Macromolecule #1: Envelope glycoprotein L
+Macromolecule #2: Envelope protein UL128
+Macromolecule #3: Envelope glycoprotein UL130
+Macromolecule #4: UL131A
+Macromolecule #5: 2-25 Fab Heavy Chain
+Macromolecule #6: 2-25 Fab Light Chain
+Macromolecule #7: 1-32 Fab Heavy Chain
+Macromolecule #8: 1-32 Fab Light Chain
+Macromolecule #9: 1-103 Fab Heavy Chain
+Macromolecule #10: 1-103 Fab Light Chain
+Macromolecule #11: 2-acetamido-2-deoxy-beta-D-glucopyranose
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: PLASMA CLEANING | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV Details: Blotted for six seconds with a force of "1" before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 36.0 e/Å2 Details: Collected from a single grid at a mixture of 0 degrees tilt and -30 degrees tilt |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)