+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22455 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Adeno-Associated Virus 2 Rep68 HD Hexamer-ssDNA with ATPgS | |||||||||
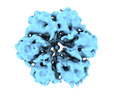
 Map data Map data | Local Refinement of helicase domain hexamer bound to ssDNA | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | AAV / Protein-DNA / AAA+ / SF3 / HUH / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated arrest of host cell cycle during G2/M transition / symbiont entry into host cell via permeabilization of host membrane / viral DNA genome replication / symbiont-mediated perturbation of host cell cycle G1/S transition checkpoint / endonuclease activity / DNA helicase / DNA replication / host cell nucleus / ATP hydrolysis activity / DNA binding ...symbiont-mediated arrest of host cell cycle during G2/M transition / symbiont entry into host cell via permeabilization of host membrane / viral DNA genome replication / symbiont-mediated perturbation of host cell cycle G1/S transition checkpoint / endonuclease activity / DNA helicase / DNA replication / host cell nucleus / ATP hydrolysis activity / DNA binding / ATP binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  Adeno-associated virus - 2 / synthetic construct (others) Adeno-associated virus - 2 / synthetic construct (others) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.01 Å | |||||||||
 Authors Authors | Escalante CR | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2020 Journal: Nucleic Acids Res / Year: 2020Title: The Cryo-EM structure of AAV2 Rep68 in complex with ssDNA reveals a malleable AAA+ machine that can switch between oligomeric states. Authors: Vishaka Santosh / Faik N Musayev / Rahul Jaiswal / Francisco Zárate-Pérez / Bram Vandewinkel / Caroline Dierckx / Molly Endicott / Kamyar Sharifi / Kelly Dryden / Els Henckaerts / Carlos R Escalante /   Abstract: The adeno-associated virus (AAV) non-structural Rep proteins catalyze all the DNA transactions required for virus viability including, DNA replication, transcription regulation, genome packaging, and ...The adeno-associated virus (AAV) non-structural Rep proteins catalyze all the DNA transactions required for virus viability including, DNA replication, transcription regulation, genome packaging, and during the latent phase, site-specific integration. Rep proteins contain two multifunctional domains: an Origin Binding Domain (OBD) and a SF3 helicase domain (HD). Studies have shown that Rep proteins have a dynamic oligomeric behavior where the nature of the DNA substrate molecule modulates its oligomeric state. In the presence of ssDNA, Rep68 forms a large double-octameric ring complex. To understand the mechanisms underlying AAV Rep function, we investigated the cryo-EM and X-ray structures of Rep68-ssDNA complexes. Surprisingly, Rep68 generates hybrid ring structures where the OBD forms octameric rings while the HD forms heptamers. Moreover, the binding to ATPγS promotes a large conformational change in the entire AAA+ domain that leads the HD to form both heptamer and hexamers. The HD oligomerization is driven by an interdomain linker region that acts as a latch to 'catch' the neighboring HD subunit and is flexible enough to permit the formation of different stoichiometric ring structures. Overall, our studies show the structural basis of AAV Rep's structural flexibility required to fulfill its multifunctional role during the AAV life cycle. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22455.map.gz emd_22455.map.gz | 28.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22455-v30.xml emd-22455-v30.xml emd-22455.xml emd-22455.xml | 14 KB 14 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_22455.png emd_22455.png | 119.1 KB | ||
| Filedesc metadata |  emd-22455.cif.gz emd-22455.cif.gz | 6.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22455 http://ftp.pdbj.org/pub/emdb/structures/EMD-22455 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22455 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22455 | HTTPS FTP |
-Related structure data
| Related structure data |  7jsiMC  6xb8C  7jseC  7jsfC  7jsgC  7jshC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22455.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22455.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local Refinement of helicase domain hexamer bound to ssDNA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.073 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : AAV-2 Rep68/dT25 complex
| Entire | Name: AAV-2 Rep68/dT25 complex |
|---|---|
| Components |
|
-Supramolecule #1: AAV-2 Rep68/dT25 complex
| Supramolecule | Name: AAV-2 Rep68/dT25 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: hexameric complex |
|---|---|
| Source (natural) | Organism:  Adeno-associated virus - 2 Adeno-associated virus - 2 |
| Molecular weight | Theoretical: 370 KDa |
-Macromolecule #1: Protein Rep68
| Macromolecule | Name: Protein Rep68 / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO / EC number: DNA helicase |
|---|---|
| Source (natural) | Organism:  Adeno-associated virus - 2 Adeno-associated virus - 2 |
| Molecular weight | Theoretical: 60.829742 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPGFYEIVIK VPSDLDGHLP GISDSFVNWV AEKEWELPPD SDMDLNLIEQ APLTVAEKLQ RDFLTEWRRV SKAPEALFFV QFEKGESYF HMHVLVETTG VKSMVLGRFL SQIREKLIQR IYRGIEPTLP NWFAVTKTRN GAGGGNKVVD ECYIPNYLLP K TQPELQWA ...String: MPGFYEIVIK VPSDLDGHLP GISDSFVNWV AEKEWELPPD SDMDLNLIEQ APLTVAEKLQ RDFLTEWRRV SKAPEALFFV QFEKGESYF HMHVLVETTG VKSMVLGRFL SQIREKLIQR IYRGIEPTLP NWFAVTKTRN GAGGGNKVVD ECYIPNYLLP K TQPELQWA WTNMEQYLSA CLNLTERKRL VAQHLTHVSQ TQEQNKENQN PNSDAPVIRS KTSARYMELV GWLVDKGITS EK QWIQEDQ ASYISFNAAS NSRSQIKAAL DNAGKIMSLT KTAPDYLVGQ QPVEDISSNR IYKILELNGY DPQYAASVFL GWA TKKFGK RNTIWLFGPA TTGKTNIAEA IAHTVPFYGC VNWTNENFPF NDCVDKMVIW WEEGKMTAKV VESAKAILGG SKVR VDQKC KSSAQIDPTP VIVTSNTNMC AVIDGNSTTF EHQQPLQDRM FKFELTRRLD HDFGKVTKQE VKDFFRWAKD HVVEV EHEF YVKKGGAKKR PAPSDADISE PKRVRESVAQ PSTSDAEASI NYADRLARGH SL UniProtKB: Protein Rep68 |
-Macromolecule #2: DNA (5'-D(P*TP*TP*TP*TP*TP*TP*TP*T)-3')
| Macromolecule | Name: DNA (5'-D(P*TP*TP*TP*TP*TP*TP*TP*T)-3') / type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 2.388585 KDa |
| Sequence | String: (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.9 Component:
| ||||||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Pretreatment - Atmosphere: AMYLAMINE | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 1722 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















