+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1gkc | ||||||
|---|---|---|---|---|---|---|---|
| Title | MMP9-inhibitor complex | ||||||
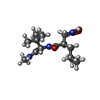
 Components Components | 92 KDA TYPE IV COLLAGENASE | ||||||
 Keywords Keywords | HYDROLASE/HYDROLASE INHIBITOR / MATRIX METALLOPROTEASE / HYDROLASE / GLYCOPROTEIN / HYDROLASE-HYDROLASE INHIBITOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationgelatinase B / negative regulation of epithelial cell differentiation involved in kidney development / : / : / cellular response to UV-A / regulation of neuroinflammatory response / positive regulation of keratinocyte migration / positive regulation of epidermal growth factor receptor signaling pathway / Assembly of collagen fibrils and other multimeric structures / positive regulation of DNA binding ...gelatinase B / negative regulation of epithelial cell differentiation involved in kidney development / : / : / cellular response to UV-A / regulation of neuroinflammatory response / positive regulation of keratinocyte migration / positive regulation of epidermal growth factor receptor signaling pathway / Assembly of collagen fibrils and other multimeric structures / positive regulation of DNA binding / Activation of Matrix Metalloproteinases / negative regulation of intrinsic apoptotic signaling pathway / endodermal cell differentiation / positive regulation of release of cytochrome c from mitochondria / Collagen degradation / response to amyloid-beta / collagen catabolic process / macrophage differentiation / extracellular matrix disassembly / EPH-ephrin mediated repulsion of cells / ephrin receptor signaling pathway / collagen binding / positive regulation of vascular associated smooth muscle cell proliferation / Degradation of the extracellular matrix / extracellular matrix organization / embryo implantation / skeletal system development / Signaling by SCF-KIT / metalloendopeptidase activity / metallopeptidase activity / positive regulation of protein phosphorylation / tertiary granule lumen / cell migration / peptidase activity / : / cellular response to lipopolysaccharide / Interleukin-4 and Interleukin-13 signaling / endopeptidase activity / ficolin-1-rich granule lumen / Extra-nuclear estrogen signaling / positive regulation of apoptotic process / serine-type endopeptidase activity / apoptotic process / Neutrophil degranulation / negative regulation of apoptotic process / proteolysis / extracellular space / extracellular exosome / extracellular region / zinc ion binding / identical protein binding Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | ||||||
 Authors Authors | Rowsell, S. / Pauptit, R.A. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2002 Journal: J.Mol.Biol. / Year: 2002Title: Crystal Structure of Mmp9 in Complex with a Reverse Hydroxamate Inhibitor Authors: Rowsell, S. / Hawtin, P. / Minshull, C.A. / Jepson, H. / Brockbank, S. / Barratt, D. / Slater, A.M. / Mcpheat, W. / Waterson, D. / Henney, A. / Pauptit, R.A. | ||||||
| History |
|
- Structure visualization
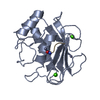
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1gkc.cif.gz 1gkc.cif.gz | 83.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1gkc.ent.gz pdb1gkc.ent.gz | 62.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1gkc.json.gz 1gkc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gk/1gkc https://data.pdbj.org/pub/pdb/validation_reports/gk/1gkc ftp://data.pdbj.org/pub/pdb/validation_reports/gk/1gkc ftp://data.pdbj.org/pub/pdb/validation_reports/gk/1gkc | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1gkdC  1hfsS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 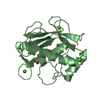
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (-0.22861, -0.9625, 0.14604), Vector: |
- Components
Components
| #1: Protein | Mass: 18384.408 Da / Num. of mol.: 2 / Fragment: CATALYTIC DOMAIN RESIDUES 107-215,391-443 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Production host: HOMO SAPIENS (human) / Production host:  #2: Chemical | ChemComp-CA / #3: Chemical | #4: Chemical | ChemComp-ZN / #5: Water | ChemComp-HOH / | Sequence details | THE INITIAL METHIONINE | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 2 X-RAY DIFFRACTION / Number of used crystals: 2 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.8 Å3/Da / Density % sol: 50 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 7.5 Details: THE CRYSTALLISATION DROPS CONTAINED A 1:1 MIXTURE OF PURIFIED COMPLEX SOLUTION (0.55 MG/ML PROTEIN AND 0.5 MM INHIBITOR SOLUTION CONCENTRATED TO ~4 MG/ML IN 20 MM TRIS- HCL PH 7.5, 2 MM ...Details: THE CRYSTALLISATION DROPS CONTAINED A 1:1 MIXTURE OF PURIFIED COMPLEX SOLUTION (0.55 MG/ML PROTEIN AND 0.5 MM INHIBITOR SOLUTION CONCENTRATED TO ~4 MG/ML IN 20 MM TRIS- HCL PH 7.5, 2 MM CACL2, 50 MM NACL) AND RESERVOIR BUFFER (3.6 M NACL, 0.1 M HEPES PH 7.5). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 15 ℃ / Method: vapor diffusion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 298 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SRS SRS  / Beamline: PX9.6 / Wavelength: 0.87 / Beamline: PX9.6 / Wavelength: 0.87 |
| Detector | Type: ADSC CCD / Detector: CCD / Date: Aug 25, 1999 / Details: SILICON MIRROR |
| Radiation | Monochromator: SI(III) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.87 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→51.3 Å / Num. obs: 17375 / % possible obs: 88 % / Redundancy: 4.5 % / Biso Wilson estimate: 28.3 Å2 / Rmerge(I) obs: 0.117 / Rsym value: 0.117 / Net I/σ(I): 14.4 |
| Reflection shell | Resolution: 2.3→2.44 Å / Redundancy: 4.4 % / Rmerge(I) obs: 0.479 / Mean I/σ(I) obs: 4.2 / Rsym value: 0.479 / % possible all: 83 |
| Reflection | *PLUS Highest resolution: 2.3 Å / % possible obs: 88.3 % / Num. measured all: 77726 |
| Reflection shell | *PLUS % possible obs: 83.3 % / Num. unique obs: 2656 / Num. measured obs: 10147 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1HFS Resolution: 2.3→51.3 Å / Rfactor Rfree error: 0.009 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 / Details: X-PLOR, CNS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: MASK / Bsol: 47.85 Å2 / ksol: 0.37 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 28 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→51.3 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS | Rms dev Biso : 2.6 Å2 / Rms dev position: 0.09 Å / Weight Biso : 2 / Weight position: 50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.3→2.38 Å / Rfactor Rfree error: 0.041 / Total num. of bins used: 10
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.3 Å / Lowest resolution: 51.3 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor obs: 0.277 |
 Movie
Movie Controller
Controller










 PDBj
PDBj