[English] 日本語
 Yorodumi
Yorodumi- PDB-1ppl: CRYSTALLOGRAPHIC ANALYSIS OF TRANSITION-STATE MIMICS BOUND TO PEN... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ppl | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTALLOGRAPHIC ANALYSIS OF TRANSITION-STATE MIMICS BOUND TO PENICILLOPEPSIN: PHOSPHORUS-CONTAINING PEPTIDE ANALOGUES | ||||||
 Components Components | PENICILLOPEPSIN | ||||||
 Keywords Keywords | HYDROLASE/hydrolase inhibitor / ACID PROTEINASE / HYDROLASE-hydrolase inhibitor complex | ||||||
| Function / homology |  Function and homology information Function and homology informationpenicillopepsin / aspartic-type endopeptidase activity / proteolysis / extracellular region Similarity search - Function | ||||||
| Biological species |  Penicillium janthinellum (fungus) Penicillium janthinellum (fungus) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.7 Å X-RAY DIFFRACTION / Resolution: 1.7 Å | ||||||
 Authors Authors | Fraser, M.E. / James, M.N.G. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 1992 Journal: Biochemistry / Year: 1992Title: Crystallographic analysis of transition-state mimics bound to penicillopepsin: phosphorus-containing peptide analogues. Authors: Fraser, M.E. / Strynadka, N.C. / Bartlett, P.A. / Hanson, J.E. / James, M.N. #1:  Journal: Biological Macromolecules and Assemblies / Year: 1987 Journal: Biological Macromolecules and Assemblies / Year: 1987Title: Aspartic Proteinases and Their Catalytic Pathway Authors: James, M.N.G. / Sielecki, A.R. #2:  Journal: Biochemistry / Year: 1985 Journal: Biochemistry / Year: 1985Title: Stereochemical Analysis of Peptide Bond Hydrolysis Catalyzed by the Aspartic Proteinase Penicillopepsin Authors: James, M.N.G. / Sielecki, A.R. #3:  Journal: J.Mol.Biol. / Year: 1983 Journal: J.Mol.Biol. / Year: 1983Title: Structure and Refinement of Penicillopepsin at 1.8 Angstroms Resolution Authors: James, M.N.G. / Sielecki, A.R. #4:  Journal: Proc.Natl.Acad.Sci.USA / Year: 1982 Journal: Proc.Natl.Acad.Sci.USA / Year: 1982Title: Conformational Flexibility in the Active Sites of Aspartyl Proteinases Revealed by a Pepstatin Fragment Binding to Penicillopepsin Authors: James, M.N.G. / Sielecki, A. / Salituro, F. / Rich, D.H. / Hofmann, T. #5:  Journal: STRUCTURAL STUDIES ON MOLECULES OF BIOLOGICA INTERESTL Journal: STRUCTURAL STUDIES ON MOLECULES OF BIOLOGICA INTERESTLYear: 1981 Title: The Tertiary Structure of Penicillopepsin. Towards a Catalytic Mechanism for Acid Proteases Authors: James, M.N.G. / Hsu, I-N. / Hofmann, T. / Sielecki, A.R. #6:  Journal: Can.J.Biochem. / Year: 1980 Journal: Can.J.Biochem. / Year: 1980Title: An X-Ray Crystallographic Approach to Enzyme Structure and Function Authors: James, M.N.G. #7:  Journal: Nature / Year: 1978 Journal: Nature / Year: 1978Title: Structural Evidence for Gene Duplication in the Evolution of the Acid Proteases Authors: Tang, J. / James, M.N.G. / Hsu, I.N. / Jenkins, J.A. / Blundell, T.L. #8:  Journal: Nature / Year: 1977 Journal: Nature / Year: 1977Title: Mechanism of Acid Protease Catalysis Based on the Crystal Structure of Penicillopepsin Authors: James, M.N.G. / Hsu, I.-N. / Delbaere, L.T.J. #9:  Journal: Nature / Year: 1977 Journal: Nature / Year: 1977Title: Penicillopepsin from Penicillium Janthinellum Crystal Structure at 2.8 Angstroms and Sequence Homology with Porcine Pepsin Authors: Hsu, I.-N. / Delbaere, L.T.J. / James, M.N.G. / Hofmann, T. #10:  Journal: Adv.Exp.Med.Biol. / Year: 1977 Journal: Adv.Exp.Med.Biol. / Year: 1977Title: Penicillopepsin. 2.8 Angstroms Structure, Active Site Conformation and Mechanistic Implications Authors: Hsu, I-N. / Delbaere, L.T.J. / James, M.N.G. / Hofmann, T. #11:  Journal: Biochem.Biophys.Res.Commun. / Year: 1976 Journal: Biochem.Biophys.Res.Commun. / Year: 1976Title: The Crystal Structure of Penicillopepsin at 6 Angstroms Resolution Authors: Hsu, I-N. / Hofmann, T. / Nyburg, S.C. / James, M.N.G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ppl.cif.gz 1ppl.cif.gz | 82 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ppl.ent.gz pdb1ppl.ent.gz | 59.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ppl.json.gz 1ppl.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1ppl_validation.pdf.gz 1ppl_validation.pdf.gz | 509.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1ppl_full_validation.pdf.gz 1ppl_full_validation.pdf.gz | 520.2 KB | Display | |
| Data in XML |  1ppl_validation.xml.gz 1ppl_validation.xml.gz | 10 KB | Display | |
| Data in CIF |  1ppl_validation.cif.gz 1ppl_validation.cif.gz | 16.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pp/1ppl https://data.pdbj.org/pub/pdb/validation_reports/pp/1ppl ftp://data.pdbj.org/pub/pdb/validation_reports/pp/1ppl ftp://data.pdbj.org/pub/pdb/validation_reports/pp/1ppl | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| Unit cell |
| |||||||||
| Atom site foot note | 1: RESIDUES 134 AND 315 ARE CIS PROLINES. | |||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules E
| #1: Protein | Mass: 33468.809 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Penicillium janthinellum (fungus) / References: UniProt: P00798, penicillopepsin Penicillium janthinellum (fungus) / References: UniProt: P00798, penicillopepsin |
|---|
-Sugars , 2 types, 2 molecules 
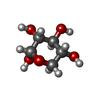

| #3: Sugar | ChemComp-MAN / |
|---|---|
| #4: Sugar | ChemComp-XYS / |
-Non-polymers , 3 types, 278 molecules 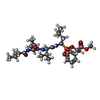




| #2: Chemical | ChemComp-1Z7 / |
|---|---|
| #5: Chemical | ChemComp-SO4 / |
| #6: Water | ChemComp-HOH / |
-Details
| Has protein modification | Y |
|---|---|
| Nonpolymer details | THE INHIBITOR SUBCOMPONENT ZPH HAS A MODIFIED LEUCINE IN WHICH THE CO GROUP HAS BEEN REPLACED WITH ...THE INHIBITOR SUBCOMPONE |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.98 Å3/Da / Density % sol: 37.96 % | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS Temperature: 18-22 ℃ / pH: 4.4 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Highest resolution: 1.7 Å / Lowest resolution: 60 Å / Num. all: 29601 / Num. obs: 26533 / Num. measured all: 88917 / Rmerge(I) obs: 0.06 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Rfactor Rwork: 0.148 / Rfactor obs: 0.148 / Highest resolution: 1.7 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Highest resolution: 1.7 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 1.7 Å / Lowest resolution: 8 Å / Num. reflection obs: 24760 / σ(F): 3 / Rfactor obs: 0.148 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller























 PDBj
PDBj







