+Search query
-Structure paper
| Title | Targeting ryanodine receptors with allopurinol and xanthine derivatives for the treatment of cardiac and musculoskeletal weakness disorders. |
|---|---|
| Journal, issue, pages | Proc Natl Acad Sci U S A, Vol. 122, Issue 24, Page e2422082122, Year 2025 |
| Publish date | Jun 17, 2025 |
 Authors Authors | Marco C Miotto / Estefania Luna-Figueroa / Carl Tchagou / Laith Bahlouli / Steven Reiken / Haikel Dridi / Yang Liu / Gunnar Weninger / Andrew R Marks /  |
| PubMed Abstract | Ryanodine receptors (RyRs) are intracellular Ca channels essential for muscle contraction. Caffeine, a xanthine derivative, has been known for decades to increase muscle contraction and enhance ...Ryanodine receptors (RyRs) are intracellular Ca channels essential for muscle contraction. Caffeine, a xanthine derivative, has been known for decades to increase muscle contraction and enhance activation of RyRs by increasing the sensitivity to Ca. We previously showed that xanthine, the only physiologically relevant xanthine derivative, also binds to and activates RyR2. Most xanthine derivatives and analogs are safe and widely prescribed, with the most popular being the xanthine oxidoreductase inhibitor allopurinol (~15M yearly prescriptions in USA). We propose that xanthine derivatives and analogs that enhance RyRs activity could be used for lead optimization and eventually for the treatment of the diseases that exhibit decreased muscle contraction and reduced RyRs activity, such as RyR1-related diseases, sarcopenia, and heart failure. Here, we show by cryo-EM that xanthine derivatives, analogs, and other related compounds bind to the xanthine/caffeine binding site and activate RyR1, and identify 4-oxopyrimidine as the minimal motif necessary for such interaction. |
 External links External links |  Proc Natl Acad Sci U S A / Proc Natl Acad Sci U S A /  PubMed:40512792 / PubMed:40512792 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.12 - 4.49 Å |
| Structure data |  EMDB-47336: Structure of PKA phosphorylated human RyR2-R2474S in the open state in the presence of Calmodulin - focused map on xanthine  EMDB-47357: Structure of RyR1 in the primed state in the presence of caffeine - focused map (reprocessed from EMPIAR-10997) EMDB-47385, PDB-9e18: EMDB-47386, PDB-9e19: EMDB-47387, PDB-9e1a: EMDB-47388, PDB-9e1b: EMDB-47389, PDB-9e1c: EMDB-47390, PDB-9e1d: EMDB-47391, PDB-9e1e: EMDB-47392, PDB-9e1f: EMDB-47393, PDB-9e1g: EMDB-47394, PDB-9e1h: EMDB-47395, PDB-9e1i:  EMDB-47396: Structure of RyR1 in the primed state in the presence of pentoxifylline, focused refinement  EMDB-47397: Structure of RyR1 in the open state in the presence of pentoxifylline, focused refinement  EMDB-47398: Structure of RyR1 in the primed state in the presence of dyphylline, focused refinement  EMDB-47399: Structure of RyR1 in the open state in the presence of dyphylline, focused refinement  EMDB-47400: Structure of RyR1 in the primed state in the presence of IBMX, focused refinement  EMDB-47401: Structure of RyR1 in the primed state in the presence of enprofylline, focused refinement  EMDB-47402: Structure of RyR1 in the primed state in the presence of uracil, focused refinement  EMDB-47403: Structure of RyR1 in the primed state in the presence of allopurinol, focused refinement  EMDB-47404: Structure of RyR1 in the primed state in the presence of oxypurinol, focused refinement  EMDB-47405: Structure of RyR1 in the primed state in the presence of oxopyricid, focused refinement  EMDB-47406: Structure of RyR1 in the open state in the presence of oxopyricid, focused refinement  PDB-9e17: |
| Chemicals |  ChemComp-CA:  ChemComp-ATP:  ChemComp-ZN:  ChemComp-CFF: 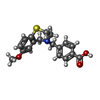 ChemComp-KVR:  ChemComp-L9R:  ChemComp-HOH: 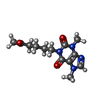 ChemComp-PNX:  PDB-1bd2:  ChemComp-IBM:  PDB-1bd3: 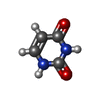 ChemComp-URA:  PDB-1bd4: 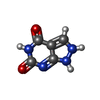 ChemComp-141:  PDB-1bd5: |
| Source |
|
 Keywords Keywords | TRANSPORT PROTEIN / calcium channel / sarcoplasmic reticulum |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers

























 homo sapiens (human)
homo sapiens (human)
