+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-30369 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Cryo-EM structure of E.coli MlaFEB with AMPPNP | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | Mla complex / Lipid transporter / MEMBRANE PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報ATPase-coupled transmembrane transporter activity / ATP-binding cassette (ABC) transporter complex / ATP binding 類似検索 - 分子機能 | |||||||||
| 生物種 |   | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.4 Å | |||||||||
 データ登録者 データ登録者 | Zhou C / Shi H | |||||||||
 引用 引用 |  ジャーナル: J Mol Biol / 年: 2021 ジャーナル: J Mol Biol / 年: 2021タイトル: Structural Insight into Phospholipid Transport by the MlaFEBD Complex from P. aeruginosa. 著者: Changping Zhou / Huigang Shi / Manfeng Zhang / Lijun Zhou / Le Xiao / Shasha Feng / Wonpil Im / Min Zhou / Xinzheng Zhang / Yihua Huang /   要旨: The outer membrane (OM) of Gram-negative bacteria, which consists of lipopolysaccharides (LPS) in the outer leaflet and phospholipids (PLs) in the inner leaflet, plays a key role in antibiotic ...The outer membrane (OM) of Gram-negative bacteria, which consists of lipopolysaccharides (LPS) in the outer leaflet and phospholipids (PLs) in the inner leaflet, plays a key role in antibiotic resistance and pathogen virulence. The maintenance of lipid asymmetry (Mla) pathway is known to be involved in PL transport and contributes to the lipid homeostasis of the OM, yet the underlying molecular mechanism and the directionality of PL transport in this pathway remain elusive. Here, we reported the cryo-EM structures of the ATP-binding cassette (ABC) transporter MlaFEBD from P. areuginosa, the core complex in the Mla pathway, in nucleotide-free (apo)-, ADP (ATP + vanadate)- and ATP (AMPPNP)-bound states as well as the structures of MlaFEB from E. coli in apo- and AMPPNP-bound states at a resolution range of 3.4-3.9 Å. The structures show that the MlaFEBD complex contains a total of twelve protein molecules with a stoichiometry of MlaFEBD, and binds a plethora of PLs at different locations. In contrast to canonical ABC transporters, nucleotide binding fails to trigger significant conformational changes of both MlaFEBD and MlaFEB in the nucleotide-binding and transmembrane domains of the ABC transporter, correlated with their low ATPase activities exhibited in both detergent micelles and lipid nanodiscs. Intriguingly, PLs or detergents appeared to relocate to the membrane-proximal end from the distal end of the hydrophobic tunnel formed by the MlaD hexamer in MlaFEBD upon addition of ATP, indicating that retrograde PL transport might occur in the tunnel in an ATP-dependent manner. Site-specific photocrosslinking experiment confirms that the substrate-binding pocket in the dimeric MlaE and the MlaD hexamer are able to bind PLs in vitro, in line with the notion that MlaFEBD complex functions as a PL transporter. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_30369.map.gz emd_30369.map.gz | 59.9 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-30369-v30.xml emd-30369-v30.xml emd-30369.xml emd-30369.xml | 16.3 KB 16.3 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
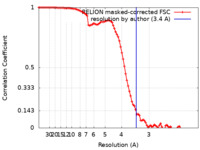
| FSC (解像度算出) |  emd_30369_fsc.xml emd_30369_fsc.xml | 9.1 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_30369.png emd_30369.png | 71.2 KB | ||
| Filedesc metadata |  emd-30369.cif.gz emd-30369.cif.gz | 6 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30369 http://ftp.pdbj.org/pub/emdb/structures/EMD-30369 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30369 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30369 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_30369_validation.pdf.gz emd_30369_validation.pdf.gz | 569 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_30369_full_validation.pdf.gz emd_30369_full_validation.pdf.gz | 568.6 KB | 表示 | |
| XML形式データ |  emd_30369_validation.xml.gz emd_30369_validation.xml.gz | 10.8 KB | 表示 | |
| CIF形式データ |  emd_30369_validation.cif.gz emd_30369_validation.cif.gz | 14.2 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30369 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30369 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30369 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30369 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_30369.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_30369.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : E.coli MlaFEB bond with AMPPNP
| 全体 | 名称: E.coli MlaFEB bond with AMPPNP |
|---|---|
| 要素 |
|
-超分子 #1: E.coli MlaFEB bond with AMPPNP
| 超分子 | 名称: E.coli MlaFEB bond with AMPPNP / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#3 |
|---|---|
| 由来(天然) | 生物種:  |
-分子 #1: Lipid asymmetry maintenance ABC transporter permease subunit MlaE
| 分子 | 名称: Lipid asymmetry maintenance ABC transporter permease subunit MlaE タイプ: protein_or_peptide / ID: 1 / コピー数: 2 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 27.885162 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MLLNALASLG HKGIKTLRTF GRAGLMLFNA LVGKPEFRKH APLLVRQLYN VGVLSMLIIV VSGVFIGMVL GLQGYLVLTT YSAETSLGM LVALSLLREL GPVVAALLFA GRAGSALTAE IGLMRATEQL SSMEMMAVDP LRRVISPRFW AGVISLPLLT V IFVAVGIW ...文字列: MLLNALASLG HKGIKTLRTF GRAGLMLFNA LVGKPEFRKH APLLVRQLYN VGVLSMLIIV VSGVFIGMVL GLQGYLVLTT YSAETSLGM LVALSLLREL GPVVAALLFA GRAGSALTAE IGLMRATEQL SSMEMMAVDP LRRVISPRFW AGVISLPLLT V IFVAVGIW GGSLVGVSWK GIDSGFFWSA MQNAVDWRMD LVNCLIKSVV FAITVTWISL FNGYDAIPTS AGISRATTRT VV HSSLAVL GLDFVLTALM FGN UniProtKB: Intermembrane phospholipid transport system permease protein MlaE |
-分子 #2: Phospholipid ABC transporter ATP-binding protein MlaF
| 分子 | 名称: Phospholipid ABC transporter ATP-binding protein MlaF タイプ: protein_or_peptide / ID: 2 / コピー数: 2 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 29.128801 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MEQSVANLVD MRDVSFTRGN RCIFDNISLT VPRGKITAIM GPSGIGKTTL LRLIGGQIAP DHGEILFDGE NIPAMSRSRL YTVRKRMSM LFQSGALFTD MNVFDNVAYP LREHTQLPAP LLHSTVMMKL EAVGLRGAAK LMPSELSGGM ARRAALARAI A LEPDLIMF ...文字列: MEQSVANLVD MRDVSFTRGN RCIFDNISLT VPRGKITAIM GPSGIGKTTL LRLIGGQIAP DHGEILFDGE NIPAMSRSRL YTVRKRMSM LFQSGALFTD MNVFDNVAYP LREHTQLPAP LLHSTVMMKL EAVGLRGAAK LMPSELSGGM ARRAALARAI A LEPDLIMF DEPFVGQDPI TMGVLVKLIS ELNSALGVTC VVVSHDVPEV LSIADHAWIL ADKKIVAHGS AQALQANPDP RV RQFLDGI ADGPVPFRYP AGDYHADLLP GS UniProtKB: Phospholipid ABC transporter ATP-binding protein MlaF |
-分子 #3: Lipid asymmetry maintenance protein MlaB
| 分子 | 名称: Lipid asymmetry maintenance protein MlaB / タイプ: protein_or_peptide / ID: 3 / コピー数: 2 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 10.690313 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MSESLSWMQT GDTLALSGEL DQDVLLPLWE MREEAVKGIT CIDLSRVSRV DTGGLALLLH LIDLAKKQGN NVTLQGVNDK VYTLAKLYN LPADVLPR UniProtKB: Lipid asymmetry maintenance protein MlaB |
-分子 #4: PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER
| 分子 | 名称: PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER / タイプ: ligand / ID: 4 / コピー数: 2 / 式: ANP |
|---|---|
| 分子量 | 理論値: 506.196 Da |
| Chemical component information |  ChemComp-ANP: |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 8 |
|---|---|
| グリッド | モデル: Quantifoil R1.2/1.3 / 材質: COPPER / メッシュ: 300 |
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K2 QUANTUM (4k x 4k) 検出モード: SUPER-RESOLUTION / 平均電子線量: 60.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源: TUNGSTEN HAIRPIN |
| 電子光学系 | C2レンズ絞り径: 50.0 µm / 照射モード: SPOT SCAN / 撮影モード: DIFFRACTION / Cs: 2.7 mm |
| 試料ステージ | 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー



 UCSF Chimera
UCSF Chimera






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)