+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3nmu | ||||||
|---|---|---|---|---|---|---|---|
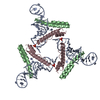
| Title | Crystal Structure of substrate-bound halfmer box C/D RNP | ||||||
 Components Components |
| ||||||
 Keywords Keywords | Transferase/RNA / Kink-turn motif / RNA assembly motif / Transferase-RNA complex | ||||||
| Function / homology |  Function and homology information Function and homology informationhistone H2AQ104 methyltransferase activity / box C/D sno(s)RNA 3'-end processing / rRNA methyltransferase activity / box C/D methylation guide snoRNP complex / ribonuclease P activity / tRNA 5'-leader removal / tRNA processing / snoRNA binding / Transferases; Transferring one-carbon groups; Methyltransferases / rRNA processing ...histone H2AQ104 methyltransferase activity / box C/D sno(s)RNA 3'-end processing / rRNA methyltransferase activity / box C/D methylation guide snoRNP complex / ribonuclease P activity / tRNA 5'-leader removal / tRNA processing / snoRNA binding / Transferases; Transferring one-carbon groups; Methyltransferases / rRNA processing / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / RNA binding / cytoplasm Similarity search - Function | ||||||
| Biological species |   Pyrococcus furiosus (archaea) Pyrococcus furiosus (archaea) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.729 Å MOLECULAR REPLACEMENT / Resolution: 2.729 Å | ||||||
 Authors Authors | Li, H. / Xue, S. / Wang, R. | ||||||
 Citation Citation |  Journal: Mol.Cell / Year: 2010 Journal: Mol.Cell / Year: 2010Title: Structural basis for substrate placement by an archaeal box C/D ribonucleoprotein particle. Authors: Xue, S. / Wang, R. / Yang, F. / Terns, R.M. / Terns, M.P. / Zhang, X. / Maxwell, E.S. / Li, H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3nmu.cif.gz 3nmu.cif.gz | 344.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3nmu.ent.gz pdb3nmu.ent.gz | 275.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3nmu.json.gz 3nmu.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/nm/3nmu https://data.pdbj.org/pub/pdb/validation_reports/nm/3nmu ftp://data.pdbj.org/pub/pdb/validation_reports/nm/3nmu ftp://data.pdbj.org/pub/pdb/validation_reports/nm/3nmu | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 3 types, 6 molecules ABCGFJ
| #1: Protein | Mass: 43937.555 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Pyrococcus furiosus (archaea) / Gene: PF0060 / Production host: Pyrococcus furiosus (archaea) / Gene: PF0060 / Production host:  #2: Protein | Mass: 14243.545 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Pyrococcus furiosus (archaea) / Gene: rpl7ae, PF1367 / Production host: Pyrococcus furiosus (archaea) / Gene: rpl7ae, PF1367 / Production host:  #4: Protein | Mass: 26760.844 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Pyrococcus furiosus (archaea) / Gene: flpA, PF0059 / Production host: Pyrococcus furiosus (archaea) / Gene: flpA, PF0059 / Production host:  References: UniProt: Q8U4M2, Transferases; Transferring one-carbon groups; Methyltransferases |
|---|
-RNA chain , 2 types, 4 molecules DEIK
| #3: RNA chain | Mass: 11009.606 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Details: box C/D RNA / Source: (gene. exp.)   Pyrococcus furiosus (archaea) Pyrococcus furiosus (archaea)#5: RNA chain | Mass: 4156.542 Da / Num. of mol.: 2 / Source method: obtained synthetically / Details: The RNA was chemically synthesized |
|---|
-Non-polymers , 2 types, 29 molecules 


| #6: Chemical | | #7: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.4 Å3/Da / Density % sol: 63.86 % |
|---|---|
| Crystal grow | Temperature: 303 K / Method: vapor diffusion, hanging drop / pH: 7 Details: The complex was equilibrated with reservoir solutions containing 0-400 mM potassium chloride, 200 mM-1.5 M sodium chloride, 150-250 mM magnesium acetate, 200 mM ammonium acetate, 50 mM HEPES- ...Details: The complex was equilibrated with reservoir solutions containing 0-400 mM potassium chloride, 200 mM-1.5 M sodium chloride, 150-250 mM magnesium acetate, 200 mM ammonium acetate, 50 mM HEPES-NaOH (pH 7.0), and 0 5% PEG 4000, VAPOR DIFFUSION, HANGING DROP, temperature 303K |
-Data collection
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 22-ID / Wavelength: 1 Å / Beamline: 22-ID / Wavelength: 1 Å |
|---|---|
| Detector | Type: MARMOSAIC 300 mm CCD / Detector: CCD |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.7→50 Å / Num. all: 63177 / Num. obs: 61445 / % possible obs: 89.2 % / Observed criterion σ(F): 2 / Observed criterion σ(I): 3 / Redundancy: 5.8 % / Biso Wilson estimate: 56.33 Å2 / Rsym value: 0.074 |
| Reflection shell | Resolution: 2.7→2.8 Å / % possible all: 58.1 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 2.729→33.572 Å / SU ML: 0.34 / σ(F): 0 / Phase error: 34.75 / Stereochemistry target values: ML MOLECULAR REPLACEMENT / Resolution: 2.729→33.572 Å / SU ML: 0.34 / σ(F): 0 / Phase error: 34.75 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 45.602 Å2 / ksol: 0.297 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.729→33.572 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj































