[日本語] English
 万見
万見- EMDB-20572: Cryo-EM structure of extracellular dimeric complex of RET/GFRAL/GDF15 -
+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-20572 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
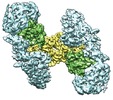
| タイトル | Cryo-EM structure of extracellular dimeric complex of RET/GFRAL/GDF15 | |||||||||
 マップデータ マップデータ | Cryo-EM map of the extracellular complex of RET/GFRAL/GDF15. C2 symmetry was applied in the 3D refinement. | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | RET / receptor tyrosine kinase / cryo-EM / SIGNALING PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報response to metformin / negative regulation of growth hormone receptor signaling pathway / glial cell-derived neurotrophic factor receptor activity / reduction of food intake in response to dietary excess / Peyer's patch morphogenesis / GDF15-GFRAL signaling pathway / positive regulation of metanephric glomerulus development / ureter maturation / embryonic epithelial tube formation / glial cell-derived neurotrophic factor receptor signaling pathway ...response to metformin / negative regulation of growth hormone receptor signaling pathway / glial cell-derived neurotrophic factor receptor activity / reduction of food intake in response to dietary excess / Peyer's patch morphogenesis / GDF15-GFRAL signaling pathway / positive regulation of metanephric glomerulus development / ureter maturation / embryonic epithelial tube formation / glial cell-derived neurotrophic factor receptor signaling pathway / lymphocyte migration into lymphoid organs / posterior midgut development / membrane protein proteolysis / Formation of the ureteric bud / positive regulation of neuron maturation / neuron cell-cell adhesion / negative regulation of leukocyte migration / Formation of the nephric duct / enteric nervous system development / negative regulation of appetite / positive regulation of fatty acid oxidation / cellular response to chemical stress / innervation / plasma membrane protein complex / positive regulation of extrinsic apoptotic signaling pathway in absence of ligand / neuron maturation / stress-activated protein kinase signaling cascade / positive regulation of cell adhesion mediated by integrin / neural crest cell migration / ureteric bud development / negative regulation of multicellular organism growth / positive regulation of myoblast fusion / regulation of axonogenesis / response to pain / negative regulation of SMAD protein signal transduction / homophilic cell adhesion via plasma membrane adhesion molecules / RET signaling / negative regulation of extrinsic apoptotic signaling pathway in absence of ligand / positive regulation of cell size / regulation of cell adhesion / cellular response to retinoic acid / transmembrane receptor protein tyrosine kinase activity / NPAS4 regulates expression of target genes / transforming growth factor beta receptor signaling pathway / axon guidance / : / cytokine activity / growth factor activity / negative regulation of transforming growth factor beta receptor signaling pathway / placental growth factor receptor activity / insulin receptor activity / vascular endothelial growth factor receptor activity / hepatocyte growth factor receptor activity / macrophage colony-stimulating factor receptor activity / platelet-derived growth factor alpha-receptor activity / platelet-derived growth factor beta-receptor activity / stem cell factor receptor activity / boss receptor activity / protein tyrosine kinase collagen receptor activity / brain-derived neurotrophic factor receptor activity / GPI-linked ephrin receptor activity / transmembrane-ephrin receptor activity / epidermal growth factor receptor activity / fibroblast growth factor receptor activity / insulin-like growth factor receptor activity / receptor protein-tyrosine kinase / hormone activity / receptor tyrosine kinase binding / positive regulation of neuron projection development / cell surface receptor protein tyrosine kinase signaling pathway / MAPK cascade / actin cytoskeleton / cell-cell signaling / signaling receptor activity / nervous system development / retina development in camera-type eye / protein tyrosine kinase activity / RAF/MAP kinase cascade / histone H3Y41 kinase activity / histone H2AXY142 kinase activity / negative regulation of neuron apoptotic process / positive regulation of MAPK cascade / early endosome / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / receptor complex / endosome membrane / positive regulation of cell migration / protein phosphorylation / response to xenobiotic stimulus / axon / external side of plasma membrane / focal adhesion / neuronal cell body / dendrite / calcium ion binding / positive regulation of gene expression / positive regulation of DNA-templated transcription / Golgi apparatus / signal transduction / protein homodimerization activity 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 4.1 Å | |||||||||
 データ登録者 データ登録者 | Li J / Shang GJ | |||||||||
 引用 引用 |  ジャーナル: Elife / 年: 2019 ジャーナル: Elife / 年: 2019タイトル: Cryo-EM analyses reveal the common mechanism and diversification in the activation of RET by different ligands. 著者: Jie Li / Guijun Shang / Yu-Ju Chen / Chad A Brautigam / Jen Liou / Xuewu Zhang / Xiao-Chen Bai /  要旨: RET is a receptor tyrosine kinase (RTK) that plays essential roles in development and has been implicated in several human diseases. Different from most of RTKs, RET requires not only its cognate ...RET is a receptor tyrosine kinase (RTK) that plays essential roles in development and has been implicated in several human diseases. Different from most of RTKs, RET requires not only its cognate ligands but also co-receptors for activation, the mechanisms of which remain unclear due to lack of high-resolution structures of the ligand/co-receptor/receptor complexes. Here, we report cryo-EM structures of the extracellular region ternary complexes of GDF15/GFRAL/RET, GDNF/GFRα1/RET, NRTN/GFRα2/RET and ARTN/GFRα3/RET. These structures reveal that all the four ligand/co-receptor pairs, while using different atomic interactions, induce a specific dimerization mode of RET that is poised to bring the two kinase domains into close proximity for cross-phosphorylation. The NRTN/GFRα2/RET dimeric complex further pack into a tetrameric assembly, which is shown by our cell-based assays to regulate the endocytosis of RET. Our analyses therefore reveal both the common mechanism and diversification in the activation of RET by different ligands. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_20572.map.gz emd_20572.map.gz | 49.4 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-20572-v30.xml emd-20572-v30.xml emd-20572.xml emd-20572.xml | 15.9 KB 15.9 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_20572.png emd_20572.png | 249.5 KB | ||
| Filedesc metadata |  emd-20572.cif.gz emd-20572.cif.gz | 6.5 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20572 http://ftp.pdbj.org/pub/emdb/structures/EMD-20572 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20572 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20572 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_20572_validation.pdf.gz emd_20572_validation.pdf.gz | 533.4 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_20572_full_validation.pdf.gz emd_20572_full_validation.pdf.gz | 532.9 KB | 表示 | |
| XML形式データ |  emd_20572_validation.xml.gz emd_20572_validation.xml.gz | 5.8 KB | 表示 | |
| CIF形式データ |  emd_20572_validation.cif.gz emd_20572_validation.cif.gz | 6.7 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20572 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20572 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20572 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20572 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_20572.map.gz / 形式: CCP4 / 大きさ: 52.7 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_20572.map.gz / 形式: CCP4 / 大きさ: 52.7 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Cryo-EM map of the extracellular complex of RET/GFRAL/GDF15. C2 symmetry was applied in the 3D refinement. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : RET, GFRAL and GDF15 extracellular complex
| 全体 | 名称: RET, GFRAL and GDF15 extracellular complex |
|---|---|
| 要素 |
|
-超分子 #1: RET, GFRAL and GDF15 extracellular complex
| 超分子 | 名称: RET, GFRAL and GDF15 extracellular complex / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#3 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 200 kDa/nm |
-分子 #1: Growth/differentiation factor 15
| 分子 | 名称: Growth/differentiation factor 15 / タイプ: protein_or_peptide / ID: 1 / コピー数: 2 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 14.879113 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MGSSHHHHHH SSGLEVLFQG PHMARNGDHC PLGPGRCCRL HTVRASLEDL GWADWVLSPR EVQVTMCIGA CPSQFRAANM HAQIKTSLH RLKPDTVPAP CCVPASYNPM VLIQKTDTGV SLQTYDDLLA KDCHCI UniProtKB: Growth/differentiation factor 15 |
-分子 #2: GDNF family receptor alpha-like
| 分子 | 名称: GDNF family receptor alpha-like / タイプ: protein_or_peptide / ID: 2 / コピー数: 2 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 39.120598 KDa |
| 組換発現 | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 配列 | 文字列: QTNNCTYLRE QCLRDANGCK HAWRVMEDAC NDSDPGDPCK MRNSSYCNLS IQYLVESNFQ FKECLCTDDF YCTVNKLLGK KCINKSDNV KEDKFKWNLT TRSHHGFKGM WSCLEVAEAC VGDVVCNAQL ASYLKACSAN GNPCDLKQCQ AAIRFFYQNI P FNIAQMLA ...文字列: QTNNCTYLRE QCLRDANGCK HAWRVMEDAC NDSDPGDPCK MRNSSYCNLS IQYLVESNFQ FKECLCTDDF YCTVNKLLGK KCINKSDNV KEDKFKWNLT TRSHHGFKGM WSCLEVAEAC VGDVVCNAQL ASYLKACSAN GNPCDLKQCQ AAIRFFYQNI P FNIAQMLA FCDCAQSDIP CQQSKEALHS KTCAVNMVPP PTCLSVIRSC QNDELCRRHY RTFQSKCWQR VTRKCHEDEN CI STLSKQD LTCSGSDDCK AAYIDILGTV LQVQCTCRTI TQSEESLCKI FQHMLHRKSC FNYPTLSNVK GMALYTRKHA NKI TLTGFH SPFNGEVGTH HHHHHHH UniProtKB: GDNF family receptor alpha-like |
-分子 #3: Proto-oncogene tyrosine-protein kinase receptor Ret
| 分子 | 名称: Proto-oncogene tyrosine-protein kinase receptor Ret / タイプ: protein_or_peptide / ID: 3 / コピー数: 2 / 光学異性体: LEVO / EC番号: receptor protein-tyrosine kinase |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 69.100812 KDa |
| 組換発現 | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 配列 | 文字列: LYFSRDAYWE KLYVDQAAGT PLLYVHALRD APEEVPSFRL GQHLYGTYRT RLHENNWICI QEDTGLLYLN RSLDHSSWEK LSVRNHGFP LLTVYLKVFL SPTSLREGEC QWPGCARVYF SFFNTSFPAC SSLKPRELCF PETRPSFRIR ENRPPGTFHQ F RLLPVQFL ...文字列: LYFSRDAYWE KLYVDQAAGT PLLYVHALRD APEEVPSFRL GQHLYGTYRT RLHENNWICI QEDTGLLYLN RSLDHSSWEK LSVRNHGFP LLTVYLKVFL SPTSLREGEC QWPGCARVYF SFFNTSFPAC SSLKPRELCF PETRPSFRIR ENRPPGTFHQ F RLLPVQFL CPNISVAYRL LEGEGLPFRC APDSLEVSTR WALDREQREK YELVAVCTVH AGAREEVVMV PFPVTVYDED DS APTFPAG VDTASAVVEF KRKEDTVVAT LRVFDADVVP ASGELVRRYT STLLPGDTWA QQTFRVEHWP NETSVQANGS FVR ATVHDY RLVLNRNLSI SENRTMQLAV LVNDSDFQGP GAGVLLLHFN VSVLPVSLHL PSTYSLSVSR RARRFAQIGK VCVE NCQAF SGINVQYKLH SSGANCSTLG VVTSAEDTSG ILFVNDTKAL RRPKCAELHY MVVATDQQTS RQAQAQLLVT VEGSY VAEE AGCPLSCAVS KRRLECEECG GLGSPTGRCE WRQGDGKGIT RNFSTCSPST KTCPDGHCDV VETQDINICP QDCLRG SIV GGHEPGEPRG IKAGYGTCNC FPEEEKCFCE PEDIQDPLCD ELCRGTHHHH HHHH UniProtKB: Proto-oncogene tyrosine-protein kinase receptor Ret |
-分子 #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| 分子 | 名称: 2-acetamido-2-deoxy-beta-D-glucopyranose / タイプ: ligand / ID: 4 / コピー数: 14 / 式: NAG |
|---|---|
| 分子量 | 理論値: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-分子 #5: CALCIUM ION
| 分子 | 名称: CALCIUM ION / タイプ: ligand / ID: 5 / コピー数: 8 / 式: CA |
|---|---|
| 分子量 | 理論値: 40.078 Da |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 1 mg/mL |
|---|---|
| 緩衝液 | pH: 7.4 |
| グリッド | モデル: Quantifoil R1.2/1.3 / 材質: GOLD / メッシュ: 300 / 前処理 - タイプ: GLOW DISCHARGE / 前処理 - 時間: 80 sec. |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 277 K / 装置: FEI VITROBOT MARK IV |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 特殊光学系 | エネルギーフィルター - 名称: GIF Quantum LS / エネルギーフィルター - スリット幅: 20 eV |
| 撮影 | フィルム・検出器のモデル: GATAN K2 QUANTUM (4k x 4k) 検出モード: SUPER-RESOLUTION / デジタル化 - 画像ごとのフレーム数: 1-30 / 撮影したグリッド数: 1 / 平均露光時間: 15.0 sec. / 平均電子線量: 50.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | C2レンズ絞り径: 70.0 µm / 倍率(補正後): 46729 / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm |
| 試料ステージ | 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
+ 画像解析
画像解析
-原子モデル構築 1
| 精密化 | 空間: REAL / プロトコル: FLEXIBLE FIT / 温度因子: 190 |
|---|---|
| 得られたモデル |  PDB-6q2j: |
 ムービー
ムービー コントローラー
コントローラー

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















