[English] 日本語
 Yorodumi
Yorodumi- EMDB-20225: Dataset I: Sub-3 Angstrom Apoferritin Structure Determined With F... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20225 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Dataset I: Sub-3 Angstrom Apoferritin Structure Determined With Full Range of Phase Shifts Using A Single Position Of Volta Phase Plate | |||||||||||||||
 Map data Map data | sharpened full map | |||||||||||||||
 Sample Sample |
| |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.94 Å | |||||||||||||||
 Authors Authors | Li K / Sun C / Klose T / Irimia-Dominguez J / Vago FS / Vidal R / Jiang W | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: J Struct Biol / Year: 2019 Journal: J Struct Biol / Year: 2019Title: Sub-3 Å apoferritin structure determined with full range of phase shifts using a single position of volta phase plate. Authors: Kunpeng Li / Chen Sun / Thomas Klose / Jose Irimia-Dominguez / Frank S Vago / Ruben Vidal / Wen Jiang /  Abstract: Volta Phase Plate (VPP) has become an invaluable tool for cryo-EM structural determination of small protein complexes by increasing image contrast. Currently, the standard protocol of VPP usage ...Volta Phase Plate (VPP) has become an invaluable tool for cryo-EM structural determination of small protein complexes by increasing image contrast. Currently, the standard protocol of VPP usage periodically changes the VPP position to a fresh spot during data collection. Such a protocol was to target the phase shifts to a relatively narrow range (around 90°) based on the observations of increased phase shifts and image blur associated with more images taken with a single VPP position. Here, we report a 2.87 Å resolution structure of apoferritin reconstructed from a dataset collected using only a single position of VPP. The reconstruction resolution and map density features are nearly identical to the reconstruction from the control dataset collected with periodic change of VPP positions. Further experiments have verified that similar results, including a 2.5 Å resolution structure, could be obtained with a full range of phase shifts, different spots of variable phase shift increasing rates, and at different ages of the VPP post-installation. Furthermore, we have found that the phase shifts at low resolutions, probably related to the finite size of the Volta spots, could not be correctly modeled by current CTF model using a constant phase shift at all frequencies. In dataset III, severe beam tilt issue was identified but could be computationally corrected with iterative refinements. The observations in this study may provide new insights into further improvement of both the efficiency and robustness of VPP, and to help turn VPP into a plug-and-play device for high-resolution cryo-EM. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20225.map.gz emd_20225.map.gz | 168.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20225-v30.xml emd-20225-v30.xml emd-20225.xml emd-20225.xml | 14.9 KB 14.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20225_fsc.xml emd_20225_fsc.xml | 12.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_20225.png emd_20225.png | 265.3 KB | ||
| Masks |  emd_20225_msk_1.map emd_20225_msk_1.map emd_20225_msk_2.map emd_20225_msk_2.map | 178 MB 178 MB |  Mask map Mask map | |
| Others |  emd_20225_half_map_1.map.gz emd_20225_half_map_1.map.gz emd_20225_half_map_2.map.gz emd_20225_half_map_2.map.gz | 93 MB 93 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20225 http://ftp.pdbj.org/pub/emdb/structures/EMD-20225 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20225 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20225 | HTTPS FTP |
-Validation report
| Summary document |  emd_20225_validation.pdf.gz emd_20225_validation.pdf.gz | 78.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_20225_full_validation.pdf.gz emd_20225_full_validation.pdf.gz | 77.6 KB | Display | |
| Data in XML |  emd_20225_validation.xml.gz emd_20225_validation.xml.gz | 494 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20225 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20225 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20225 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20225 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10263 (Title: Sub-3 Å Apoferritin Structure Determined With Full Range of Phase Shifts Using A Single Position Of Volta Phase Plate EMPIAR-10263 (Title: Sub-3 Å Apoferritin Structure Determined With Full Range of Phase Shifts Using A Single Position Of Volta Phase PlateData size: 778.8 Data #1: Unaligned multi-frame movie of apoferritin Dataset I [micrographs - multiframe] Data #2: Unaligned multi-frame movie of apoferritin Dataset II [micrographs - multiframe] Data #3: Unaligned multi-frame movie of apoferritin Dataset III [micrographs - multiframe] Data #4: Unaligned multi-frame movie of apoferritin Dataset IV [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_20225.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20225.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened full map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.658 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
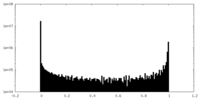
| File |  emd_20225_msk_1.map emd_20225_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Mask #2
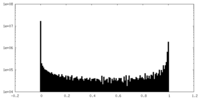
| File |  emd_20225_msk_2.map emd_20225_msk_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
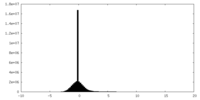
| File | emd_20225_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
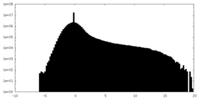
| File | emd_20225_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human Apoferritin light chain
| Entire | Name: Human Apoferritin light chain |
|---|---|
| Components |
|
-Supramolecule #1: Human Apoferritin light chain
| Supramolecule | Name: Human Apoferritin light chain / type: organelle_or_cellular_component / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 440 KDa |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Phase plate: VOLTA PHASE PLATE |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Number real images: 628 / Average electron dose: 35.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: |
|---|
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)