+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6714 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | human ferritin mutant - E-helix deletion | |||||||||
 Map data Map data | human ferritin E-helix deletion mutant | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ferritin / cage / drug delivery / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationiron ion sequestering activity / ferritin complex / Scavenging by Class A Receptors / negative regulation of ferroptosis / Golgi Associated Vesicle Biogenesis / ferroxidase / autolysosome / ferroxidase activity / negative regulation of fibroblast proliferation / ferric iron binding ...iron ion sequestering activity / ferritin complex / Scavenging by Class A Receptors / negative regulation of ferroptosis / Golgi Associated Vesicle Biogenesis / ferroxidase / autolysosome / ferroxidase activity / negative regulation of fibroblast proliferation / ferric iron binding / autophagosome / iron ion transport / Iron uptake and transport / ferrous iron binding / tertiary granule lumen / ficolin-1-rich granule lumen / intracellular iron ion homeostasis / immune response / iron ion binding / negative regulation of cell population proliferation / Neutrophil degranulation / extracellular exosome / extracellular region / identical protein binding / nucleus / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
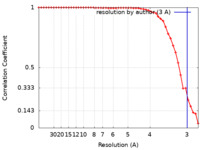
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Lee SG / Ahn BJ | |||||||||
 Citation Citation |  Journal: Angew Chem Int Ed Engl / Year: 2018 Journal: Angew Chem Int Ed Engl / Year: 2018Title: Four-fold Channel-Nicked Human Ferritin Nanocages for Active Drug Loading and pH-Responsive Drug Release. Authors: Byungjun Ahn / Seong-Gyu Lee / Hye Ryeon Yoon / Jeong Min Lee / Hyeok Jin Oh / Ho Min Kim / Yongwon Jung /  Abstract: Human ferritins are emerging platforms for non-toxic protein-based drug delivery, owing to their intrinsic or acquirable targeting abilities to cancer cells and hollow cage structures for drug ...Human ferritins are emerging platforms for non-toxic protein-based drug delivery, owing to their intrinsic or acquirable targeting abilities to cancer cells and hollow cage structures for drug loading. However, reliable strategies for high-level drug encapsulation within ferritin cavities and prompt cellular drug release are still lacking. Ferritin nanocages were developed with partially opened hydrophobic channels, which provide stable routes for spontaneous and highly accumulated loading of Fe -conjugated drugs as well as pH-responsive rapid drug release at endoplasmic pH. Multiple cancer-related compounds, such as doxorubicin, curcumin, and quercetin, were actively and heavily loaded onto the prepared nicked ferritin. Drugs on these minimally modified ferritins were effectively delivered inside cancer cells with high toxicity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6714.map.gz emd_6714.map.gz | 2.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6714-v30.xml emd-6714-v30.xml emd-6714.xml emd-6714.xml | 18.4 KB 18.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_6714_fsc.xml emd_6714_fsc.xml | 5.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_6714.png emd_6714.png | 84.8 KB | ||
| Masks |  emd_6714_msk_1.map emd_6714_msk_1.map | 8 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-6714.cif.gz emd-6714.cif.gz | 6 KB | ||
| Others |  emd_6714_half_map_1.map.gz emd_6714_half_map_1.map.gz emd_6714_half_map_2.map.gz emd_6714_half_map_2.map.gz | 4.6 MB 4.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6714 http://ftp.pdbj.org/pub/emdb/structures/EMD-6714 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6714 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6714 | HTTPS FTP |
-Related structure data
| Related structure data |  5xb1MC  6830C  5yi5C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_6714.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6714.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | human ferritin E-helix deletion mutant | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.3973 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_6714_msk_1.map emd_6714_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: human ferritin E-helix deletion mutant
| File | emd_6714_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | human ferritin E-helix deletion mutant | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: human ferritin E-helix deletion mutant
| File | emd_6714_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | human ferritin E-helix deletion mutant | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : human ferritin E-helix deletion mutant
| Entire | Name: human ferritin E-helix deletion mutant |
|---|---|
| Components |
|
-Supramolecule #1: human ferritin E-helix deletion mutant
| Supramolecule | Name: human ferritin E-helix deletion mutant / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 420 KDa |
-Macromolecule #1: Ferritin heavy chain
| Macromolecule | Name: Ferritin heavy chain / type: protein_or_peptide / ID: 1 / Number of copies: 24 / Enantiomer: LEVO / EC number: ferroxidase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 18.776082 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTTASTSQVR QNYHQDSEAA INRQINLELY ASYVYLSMSY YFDRDDVALK NFAKYFLHQS HEEREHAEKL MKLQNQRGGR IFLQDIKKP DCDDWESGLN AMECALHLEK NVNQSLLELH KLATDKNDPH LCDFIETHYL NEQVKAIKEL GDHVTNLRKM G UniProtKB: Ferritin heavy chain |
-Macromolecule #2: water
| Macromolecule | Name: water / type: ligand / ID: 2 / Number of copies: 55 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: buffers were titrated with HCl to pH8.0 | |||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: CARBON / Support film - #0 - topology: HOLEY / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: GRAPHENE OXIDE / Support film - #1 - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa Details: the grid was coated with graphene oxide prior to use | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 276 K / Instrument: FEI VITROBOT MARK IV / Details: blot for 9 seconds before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Digitization - Frames/image: 2-29 / Average exposure time: 1.8 sec. / Average electron dose: 2.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 0.002 mm / Nominal magnification: 47000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)