[English] 日本語
 Yorodumi
Yorodumi- EMDB-13844: Protein community member oxoglutarate dehydrogenase complex E2 co... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13844 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Protein community member oxoglutarate dehydrogenase complex E2 core from C. thermophilum | |||||||||
 Map data Map data | C. thermophilum Native Community Member Oxoglutarate Dehydrogenase Complex E2 Core | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Dihydrolipoyl Succinyltransferase / E2 / Oxoglutarate / a-Ketoglutarate / TRANSFERASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationL-lysine catabolic process to acetyl-CoA via saccharopine / dihydrolipoyllysine-residue succinyltransferase / dihydrolipoyllysine-residue succinyltransferase activity / oxoglutarate dehydrogenase complex / tricarboxylic acid cycle / mitochondrion Similarity search - Function | |||||||||
| Biological species |  Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) / Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) /  Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719) (fungus) Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719) (fungus) | |||||||||
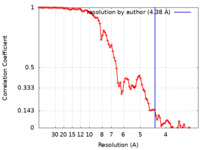
| Method | single particle reconstruction / cryo EM / Resolution: 4.38 Å | |||||||||
 Authors Authors | Skalidis I / Kyrilis FL / Tueting C / Hamdi F / Kastritis PL | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2022 Journal: Structure / Year: 2022Title: Cryo-EM and artificial intelligence visualize endogenous protein community members. Authors: Ioannis Skalidis / Fotis L Kyrilis / Christian Tüting / Farzad Hamdi / Grzegorz Chojnowski / Panagiotis L Kastritis /  Abstract: Cellular function is underlined by megadalton assemblies organizing in proximity, forming communities. Metabolons are protein communities involving metabolic pathways such as protein, fatty acid, and ...Cellular function is underlined by megadalton assemblies organizing in proximity, forming communities. Metabolons are protein communities involving metabolic pathways such as protein, fatty acid, and thioesters of coenzyme-A synthesis. Metabolons are highly heterogeneous due to their function, making their analysis particularly challenging. Here, we simultaneously characterize metabolon-embedded architectures of a 60S pre-ribosome, fatty acid synthase, and pyruvate/oxoglutarate dehydrogenase complex E2 cores de novo. Cryo-electron microscopy (cryo-EM) 3D reconstructions are resolved at 3.84-4.52 Å resolution by collecting <3,000 micrographs of a single cellular fraction. After combining cryo-EM with artificial intelligence-based atomic modeling and de novo sequence identification methods, at this resolution range, polypeptide hydrogen bonding patterns are discernible. Residing molecular components resemble their purified counterparts from other eukaryotes but also exhibit substantial conformational variation with potential functional implications. Our results propose an integrated tool, boosted by machine learning, that opens doors for structural systems biology spearheaded by cryo-EM characterization of native cell extracts. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13844.map.gz emd_13844.map.gz | 59.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13844-v30.xml emd-13844-v30.xml emd-13844.xml emd-13844.xml | 19.6 KB 19.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13844_fsc.xml emd_13844_fsc.xml | 8.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_13844.png emd_13844.png | 87.9 KB | ||
| Masks |  emd_13844_msk_1.map emd_13844_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-13844.cif.gz emd-13844.cif.gz | 5.8 KB | ||
| Others |  emd_13844_additional_1.map.gz emd_13844_additional_1.map.gz emd_13844_half_map_1.map.gz emd_13844_half_map_1.map.gz emd_13844_half_map_2.map.gz emd_13844_half_map_2.map.gz | 25.4 MB 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13844 http://ftp.pdbj.org/pub/emdb/structures/EMD-13844 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13844 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13844 | HTTPS FTP |
-Validation report
| Summary document |  emd_13844_validation.pdf.gz emd_13844_validation.pdf.gz | 1011.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_13844_full_validation.pdf.gz emd_13844_full_validation.pdf.gz | 1011.5 KB | Display | |
| Data in XML |  emd_13844_validation.xml.gz emd_13844_validation.xml.gz | 16.2 KB | Display | |
| Data in CIF |  emd_13844_validation.cif.gz emd_13844_validation.cif.gz | 20.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13844 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13844 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13844 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13844 | HTTPS FTP |
-Related structure data
| Related structure data |  7q5qMC  7q5rC  7q5sC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10892 (Title: Cryo-EM SPA dataset of Megadalton-range protein communities from a Chaetomium thermophilum native cell extract EMPIAR-10892 (Title: Cryo-EM SPA dataset of Megadalton-range protein communities from a Chaetomium thermophilum native cell extractData size: 1.1 TB Data #1: Unaligned fractions saved by Falcon 3 EC camera [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13844.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13844.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | C. thermophilum Native Community Member Oxoglutarate Dehydrogenase Complex E2 Core | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.5678 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_13844_msk_1.map emd_13844_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
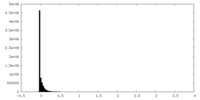
| Density Histograms |
-Additional map: C. thermophilum Native Community Member Signature 1 Ab Initio Map
| File | emd_13844_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | C. thermophilum Native Community Member Signature 1 Ab Initio Map | ||||||||||||
| Projections & Slices |
| ||||||||||||
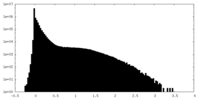
| Density Histograms |
-Half map: C. thermophilum Native Community Member Oxoglutarate Dehydrogenase Complex...
| File | emd_13844_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | C. thermophilum Native Community Member Oxoglutarate Dehydrogenase Complex E2 Core, Half-Map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
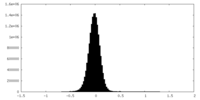
| Density Histograms |
-Half map: C. thermophilum Native Community Member Oxoglutarate Dehydrogenase Complex...
| File | emd_13844_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | C. thermophilum Native Community Member Oxoglutarate Dehydrogenase Complex E2 Core, Half-Map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
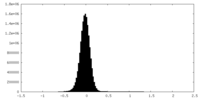
| Density Histograms |
- Sample components
Sample components
-Entire : Native 24-mer core of Oxoglutarate Dehydrogenase Complex
| Entire | Name: Native 24-mer core of Oxoglutarate Dehydrogenase Complex |
|---|---|
| Components |
|
-Supramolecule #1: Native 24-mer core of Oxoglutarate Dehydrogenase Complex
| Supramolecule | Name: Native 24-mer core of Oxoglutarate Dehydrogenase Complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) |
| Molecular weight | Theoretical: 4 MDa |
-Macromolecule #1: Dihydrolipoyllysine-residue succinyltransferase
| Macromolecule | Name: Dihydrolipoyllysine-residue succinyltransferase / type: protein_or_peptide / ID: 1 / Number of copies: 24 / Enantiomer: LEVO / EC number: dihydrolipoyllysine-residue succinyltransferase |
|---|---|
| Source (natural) | Organism:  Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719) (fungus) Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719) (fungus)Strain: DSM 1495 / CBS 144.50 / IMI 039719 |
| Molecular weight | Theoretical: 45.987004 KDa |
| Sequence | String: MLYRGLRMAA RAAPKFAVLN THAALRQLPL QFQHVRTYAD KIIKVPQMAE SITEGTLKQW NKAVGDYVEA DEEIATIETD KIDVAVNAP EAGVIKEFFV NEEDTVLVGQ DLVRLEVGGE KPAEAAKEQP KAAAPEPKVE EKKVPEAPAP EPSKTAAPAP A PPKQEAPA ...String: MLYRGLRMAA RAAPKFAVLN THAALRQLPL QFQHVRTYAD KIIKVPQMAE SITEGTLKQW NKAVGDYVEA DEEIATIETD KIDVAVNAP EAGVIKEFFV NEEDTVLVGQ DLVRLEVGGE KPAEAAKEQP KAAAPEPKVE EKKVPEAPAP EPSKTAAPAP A PPKQEAPA SPKPASKPAE TPAVTLGNRE ERRVKMNRMR LRIAERLKQS QNTAASLTTF NEVDMSALIE FRNKYKDEVL KK TGVKLGF MSAFSRAVVL AIRDLPVVNA SIEGPNGGDT IVYRDYVDIS VAVATEKGLV TPVVRNAETM DLITIEKTIA ELG KKARDG KLTIEDMAGG TFTISNGGVF GSLMGTPIIN LPQSAVLGLH AIKERPVAVN GKVEIRPMMY LALTYDHRLL DGRE AVQFL VKVKEYIEDP RKMLL UniProtKB: dihydrolipoyllysine-residue succinyltransferase |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Component - Concentration: 200.0 mM / Component - Formula: NH4CH2COOH / Component - Name: Ammonium acetate |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: For plunging, blot force 0 and blotting time of 4 sec were applied.. |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Temperature | Min: 77.15 K / Max: 103.15 K |
| Alignment procedure | Coma free - Residual tilt: 14.7 mrad |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Number real images: 2808 / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 92000 |
| Sample stage | Specimen holder model: OTHER / Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | Initial models were predicted using AlphaFold v.2.0.1, fitted into reconstructions using COOT and finally refined in real space using COOT and phenix.real_space_refine |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
| Output model |  PDB-7q5q: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)