+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13004 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Pol II-CSB-CSA-DDB1-UVSSA (Structure1) | |||||||||
 Map data Map data | Main map. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | transcription / DNA repair | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of double-strand break repair via nonhomologous end joining / regulation of transcription-coupled nucleotide-excision repair / nucleotide-excision repair complex / : / regulation of transcription elongation by RNA polymerase II / DNA protection / B-WICH complex / single strand break repair / positive regulation by virus of viral protein levels in host cell / Formation of RNA Pol II elongation complex ...negative regulation of double-strand break repair via nonhomologous end joining / regulation of transcription-coupled nucleotide-excision repair / nucleotide-excision repair complex / : / regulation of transcription elongation by RNA polymerase II / DNA protection / B-WICH complex / single strand break repair / positive regulation by virus of viral protein levels in host cell / Formation of RNA Pol II elongation complex / Formation of the Early Elongation Complex / Transcriptional regulation by small RNAs / RNA Polymerase II Pre-transcription Events / TP53 Regulates Transcription of DNA Repair Genes / FGFR2 alternative splicing / RNA polymerase II transcribes snRNA genes / mRNA Capping / mRNA Splicing - Minor Pathway / Processing of Capped Intron-Containing Pre-mRNA / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Elongation / RNA Polymerase II Transcription Initiation And Promoter Clearance / RNA Pol II CTD phosphorylation and interaction with CE / Estrogen-dependent gene expression / Formation of TC-NER Pre-Incision Complex / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / mRNA Splicing - Major Pathway / double-strand break repair via classical nonhomologous end joining / response to superoxide / epigenetic programming in the zygotic pronuclei / spindle assembly involved in female meiosis / ATP-dependent chromatin remodeler activity / photoreceptor cell maintenance / Cul4-RING E3 ubiquitin ligase complex / UV-damage excision repair / positive regulation of DNA-templated transcription, elongation / response to UV-B / RNA polymerase binding / biological process involved in interaction with symbiont / regulation of mitotic cell cycle phase transition / WD40-repeat domain binding / positive regulation of transcription by RNA polymerase III / Cul4A-RING E3 ubiquitin ligase complex / Cul4B-RING E3 ubiquitin ligase complex / ubiquitin ligase complex scaffold activity / positive regulation of nuclear-transcribed mRNA poly(A) tail shortening / organelle membrane / positive regulation of transcription by RNA polymerase I / negative regulation of reproductive process / negative regulation of developmental process / RNA polymerase II complex binding / cullin family protein binding / maintenance of transcriptional fidelity during transcription elongation by RNA polymerase II / viral release from host cell / site of DNA damage / protein tyrosine kinase activator activity / RNA Polymerase I Transcription Initiation / positive regulation of translational initiation / pyrimidine dimer repair / ATP-dependent activity, acting on DNA / positive regulation of transcription initiation by RNA polymerase II / response to X-ray / ectopic germ cell programmed cell death / transcription by RNA polymerase III / transcription by RNA polymerase I / positive regulation of double-strand break repair via homologous recombination / RNA polymerase I complex / transcription elongation by RNA polymerase I / positive regulation of viral genome replication / RNA polymerase III complex / proteasomal protein catabolic process / transcription-coupled nucleotide-excision repair / RNA polymerase II, core complex / tRNA transcription by RNA polymerase III / response to UV / : / protein autoubiquitination / JNK cascade / translation initiation factor binding / neurogenesis / positive regulation of gluconeogenesis / DNA-directed RNA polymerase activity / positive regulation of DNA repair / positive regulation of RNA splicing / DNA damage checkpoint signaling / transcription elongation factor complex / regulation of DNA-templated transcription elongation / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / response to gamma radiation / helicase activity / nucleotide-excision repair / transcription initiation at RNA polymerase II promoter / promoter-specific chromatin binding / P-body / Recognition of DNA damage by PCNA-containing replication complex / regulation of circadian rhythm / DNA Damage Recognition in GG-NER Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
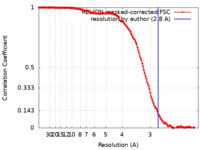
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Kokic G / Cramer P | |||||||||
| Funding support |  Germany, 2 items Germany, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2021 Journal: Nature / Year: 2021Title: Structural basis of human transcription-DNA repair coupling. Authors: Goran Kokic / Felix R Wagner / Aleksandar Chernev / Henning Urlaub / Patrick Cramer /  Abstract: Transcription-coupled DNA repair removes bulky DNA lesions from the genome and protects cells against ultraviolet (UV) irradiation. Transcription-coupled DNA repair begins when RNA polymerase II ...Transcription-coupled DNA repair removes bulky DNA lesions from the genome and protects cells against ultraviolet (UV) irradiation. Transcription-coupled DNA repair begins when RNA polymerase II (Pol II) stalls at a DNA lesion and recruits the Cockayne syndrome protein CSB, the E3 ubiquitin ligase, CRL4 and UV-stimulated scaffold protein A (UVSSA). Here we provide five high-resolution structures of Pol II transcription complexes containing human transcription-coupled DNA repair factors and the elongation factors PAF1 complex (PAF) and SPT6. Together with biochemical and published data, the structures provide a model for transcription-repair coupling. Stalling of Pol II at a DNA lesion triggers replacement of the elongation factor DSIF by CSB, which binds to PAF and moves upstream DNA to SPT6. The resulting elongation complex, EC, uses the CSA-stimulated translocase activity of CSB to pull on upstream DNA and push Pol II forward. If the lesion cannot be bypassed, CRL4 spans over the Pol II clamp and ubiquitylates the RPB1 residue K1268, enabling recruitment of TFIIH to UVSSA and DNA repair. Conformational changes in CRL4 lead to ubiquitylation of CSB and to release of transcription-coupled DNA repair factors before transcription may continue over repaired DNA. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13004.map.gz emd_13004.map.gz | 114.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13004-v30.xml emd-13004-v30.xml emd-13004.xml emd-13004.xml | 65.8 KB 65.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13004_fsc.xml emd_13004_fsc.xml | 14.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_13004.png emd_13004.png | 159.2 KB | ||
| Masks |  emd_13004_msk_1.map emd_13004_msk_1.map emd_13004_msk_2.map emd_13004_msk_2.map emd_13004_msk_3.map emd_13004_msk_3.map emd_13004_msk_4.map emd_13004_msk_4.map emd_13004_msk_5.map emd_13004_msk_5.map | 244.1 MB 244.1 MB 244.1 MB 244.1 MB 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-13004.cif.gz emd-13004.cif.gz | 13.1 KB | ||
| Others |  emd_13004_additional_1.map.gz emd_13004_additional_1.map.gz emd_13004_additional_10.map.gz emd_13004_additional_10.map.gz emd_13004_additional_2.map.gz emd_13004_additional_2.map.gz emd_13004_additional_3.map.gz emd_13004_additional_3.map.gz emd_13004_additional_4.map.gz emd_13004_additional_4.map.gz emd_13004_additional_5.map.gz emd_13004_additional_5.map.gz emd_13004_additional_6.map.gz emd_13004_additional_6.map.gz emd_13004_additional_7.map.gz emd_13004_additional_7.map.gz emd_13004_additional_8.map.gz emd_13004_additional_8.map.gz emd_13004_additional_9.map.gz emd_13004_additional_9.map.gz emd_13004_half_map_1.map.gz emd_13004_half_map_1.map.gz emd_13004_half_map_2.map.gz emd_13004_half_map_2.map.gz | 216.5 MB 217 MB 194.2 MB 194.6 MB 194.2 MB 194.6 MB 194.7 MB 194.6 MB 194.8 MB 194.8 MB 225.9 MB 226 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13004 http://ftp.pdbj.org/pub/emdb/structures/EMD-13004 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13004 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13004 | HTTPS FTP |
-Validation report
| Summary document |  emd_13004_validation.pdf.gz emd_13004_validation.pdf.gz | 903.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_13004_full_validation.pdf.gz emd_13004_full_validation.pdf.gz | 902.9 KB | Display | |
| Data in XML |  emd_13004_validation.xml.gz emd_13004_validation.xml.gz | 21 KB | Display | |
| Data in CIF |  emd_13004_validation.cif.gz emd_13004_validation.cif.gz | 27.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13004 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13004 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13004 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13004 | HTTPS FTP |
-Related structure data
| Related structure data |  7oo3MC  7oobC  7oopC  7opcC  7opdC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13004.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13004.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Main map. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
+Mask #1
+Mask #2
+Mask #3
+Mask #4
+Mask #5
+Additional map: Half map corresponding to the map focused refined on CSA-UVSSA.
+Additional map: Half map corresponding to the map focused refined on CSA-UVSSA.
+Additional map: Half map corresponding to the map focused refined on CSB.
+Additional map: Half map corresponding to the map focused refined on CSA-DDB1.
+Additional map: Half map corresponding to the map focused refined on CSB.
+Additional map: Half map corresponding to the map focused refined on CSA-DDB1.
+Additional map: Half map corresponding to the map focused refined on DDB1.
+Additional map: Half map corresponding to the map focused refined on DDB1.
+Additional map: Half map corresponding to the map focused refined on Pol II.
+Additional map: Half map corresponding to the map focused refined on Pol II.
+Half map: Half map corresponding to the main map.
+Half map: Half map corresponding to the main map.
- Sample components
Sample components
+Entire : RNA polymerase II elongation complex with CSB, CSA-DDB1 and UVSSA.
+Supramolecule #1: RNA polymerase II elongation complex with CSB, CSA-DDB1 and UVSSA.
+Supramolecule #2: DNA directed RNA polymerase
+Supramolecule #3: NTS, TS & RNA
+Supramolecule #4: DNA Repair proteins, CSB element and scaffold protein
+Macromolecule #1: DNA-directed RNA polymerase II subunit RPB1
+Macromolecule #2: DNA-directed RNA polymerase subunit beta
+Macromolecule #3: DNA-directed RNA polymerase II subunit RPB3
+Macromolecule #4: RPOL4c domain-containing protein
+Macromolecule #5: DNA-directed RNA polymerase II subunit E
+Macromolecule #6: DNA-directed RNA polymerase II subunit F
+Macromolecule #7: DNA-directed RNA polymerase II subunit RPB7
+Macromolecule #8: DNA-directed RNA polymerases I, II, and III subunit RPABC3
+Macromolecule #9: DNA-directed RNA polymerase II subunit RPB9
+Macromolecule #10: DNA-directed RNA polymerases I, II, and III subunit RPABC5
+Macromolecule #11: RNA_pol_L_2 domain-containing protein
+Macromolecule #12: RNA polymerase II subunit K
+Macromolecule #16: DNA excision repair protein ERCC-8
+Macromolecule #17: DNA excision repair protein ERCC-6
+Macromolecule #18: CSB element
+Macromolecule #19: UV-stimulated scaffold protein A
+Macromolecule #20: DNA damage-binding protein 1
+Macromolecule #13: NTS
+Macromolecule #14: TS
+Macromolecule #15: RNA
+Macromolecule #21: ZINC ION
+Macromolecule #22: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R2/1 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 10300 / Average electron dose: 40.4 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)



































































































































 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)