+ Open data
Open data
- Basic information
Basic information
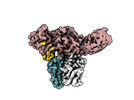
| Entry | Database: PDB / ID: 8j9k | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
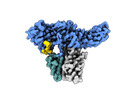
| Title | Structure of basal beta-arrestin2 | |||||||||||||||
 Components Components |
| |||||||||||||||
 Keywords Keywords |  SIGNALING PROTEIN/IMMUNE SYSTEM / SIGNALING PROTEIN/IMMUNE SYSTEM /  GPCR / GPCR /  Arrestin / Arrestin /  SIGNALING PROTEIN / SIGNALING PROTEIN /  SIGNALING PROTEIN-IMMUNE SYSTEM complex SIGNALING PROTEIN-IMMUNE SYSTEM complex | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationtype 2A serotonin receptor binding / platelet activating factor receptor binding / postsynaptic signal transduction / positive regulation of synaptic transmission, dopaminergic /  alpha-1A adrenergic receptor binding / alpha-1A adrenergic receptor binding /  follicle-stimulating hormone receptor binding / Activation of SMO / G alpha (s) signalling events / follicle-stimulating hormone receptor binding / Activation of SMO / G alpha (s) signalling events /  alpha-1B adrenergic receptor binding / follicle-stimulating hormone signaling pathway ...type 2A serotonin receptor binding / platelet activating factor receptor binding / postsynaptic signal transduction / positive regulation of synaptic transmission, dopaminergic / alpha-1B adrenergic receptor binding / follicle-stimulating hormone signaling pathway ...type 2A serotonin receptor binding / platelet activating factor receptor binding / postsynaptic signal transduction / positive regulation of synaptic transmission, dopaminergic /  alpha-1A adrenergic receptor binding / alpha-1A adrenergic receptor binding /  follicle-stimulating hormone receptor binding / Activation of SMO / G alpha (s) signalling events / follicle-stimulating hormone receptor binding / Activation of SMO / G alpha (s) signalling events /  alpha-1B adrenergic receptor binding / follicle-stimulating hormone signaling pathway / alpha-1B adrenergic receptor binding / follicle-stimulating hormone signaling pathway /  angiotensin receptor binding / positive regulation of cardiac muscle cell differentiation / WNT5A-dependent internalization of FZD4 / angiotensin receptor binding / positive regulation of cardiac muscle cell differentiation / WNT5A-dependent internalization of FZD4 /  protein kinase B binding / MAP2K and MAPK activation / Ub-specific processing proteases / negative regulation of toll-like receptor signaling pathway / Cargo recognition for clathrin-mediated endocytosis / protein kinase B binding / MAP2K and MAPK activation / Ub-specific processing proteases / negative regulation of toll-like receptor signaling pathway / Cargo recognition for clathrin-mediated endocytosis /  Clathrin-mediated endocytosis / negative regulation of interleukin-12 production / regulation of G protein-coupled receptor signaling pathway / positive regulation of calcium ion transport / arrestin family protein binding / G protein-coupled receptor internalization / type 1 angiotensin receptor binding / adult walking behavior / Thrombin signalling through proteinase activated receptors (PARs) / mitogen-activated protein kinase binding / positive regulation of epithelial cell apoptotic process / negative regulation of natural killer cell mediated cytotoxicity / negative regulation of interleukin-1 beta production / positive regulation of DNA biosynthetic process / negative regulation of release of cytochrome c from mitochondria / detection of temperature stimulus involved in sensory perception of pain / negative regulation of smooth muscle cell apoptotic process / negative regulation of interleukin-6 production / positive regulation of receptor internalization / endocytic vesicle / negative regulation of tumor necrosis factor production / Clathrin-mediated endocytosis / negative regulation of interleukin-12 production / regulation of G protein-coupled receptor signaling pathway / positive regulation of calcium ion transport / arrestin family protein binding / G protein-coupled receptor internalization / type 1 angiotensin receptor binding / adult walking behavior / Thrombin signalling through proteinase activated receptors (PARs) / mitogen-activated protein kinase binding / positive regulation of epithelial cell apoptotic process / negative regulation of natural killer cell mediated cytotoxicity / negative regulation of interleukin-1 beta production / positive regulation of DNA biosynthetic process / negative regulation of release of cytochrome c from mitochondria / detection of temperature stimulus involved in sensory perception of pain / negative regulation of smooth muscle cell apoptotic process / negative regulation of interleukin-6 production / positive regulation of receptor internalization / endocytic vesicle / negative regulation of tumor necrosis factor production /  D1 dopamine receptor binding / positive regulation of collagen biosynthetic process / negative regulation of canonical NF-kappaB signal transduction / D1 dopamine receptor binding / positive regulation of collagen biosynthetic process / negative regulation of canonical NF-kappaB signal transduction /  clathrin-coated pit / negative regulation of protein ubiquitination / cell chemotaxis / transforming growth factor beta receptor signaling pathway / 14-3-3 protein binding / negative regulation of protein phosphorylation / G protein-coupled receptor binding / clathrin-coated pit / negative regulation of protein ubiquitination / cell chemotaxis / transforming growth factor beta receptor signaling pathway / 14-3-3 protein binding / negative regulation of protein phosphorylation / G protein-coupled receptor binding /  regulation of protein phosphorylation / modulation of chemical synaptic transmission / regulation of protein phosphorylation / modulation of chemical synaptic transmission /  receptor internalization / receptor internalization /  endocytosis / positive regulation of peptidyl-tyrosine phosphorylation / endocytosis / positive regulation of peptidyl-tyrosine phosphorylation /  protein transport / positive regulation of peptidyl-serine phosphorylation / cytoplasmic vesicle / protein transport / positive regulation of peptidyl-serine phosphorylation / cytoplasmic vesicle /  postsynaptic membrane / proteasome-mediated ubiquitin-dependent protein catabolic process / basolateral plasma membrane / negative regulation of neuron apoptotic process / postsynaptic membrane / proteasome-mediated ubiquitin-dependent protein catabolic process / basolateral plasma membrane / negative regulation of neuron apoptotic process /  dendritic spine / transcription by RNA polymerase II / positive regulation of ERK1 and ERK2 cascade / molecular adaptor activity / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / protein ubiquitination / dendritic spine / transcription by RNA polymerase II / positive regulation of ERK1 and ERK2 cascade / molecular adaptor activity / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / protein ubiquitination /  endosome / positive regulation of protein phosphorylation / G protein-coupled receptor signaling pathway / protein domain specific binding / endosome / positive regulation of protein phosphorylation / G protein-coupled receptor signaling pathway / protein domain specific binding /  signaling receptor binding / glutamatergic synapse / signaling receptor binding / glutamatergic synapse /  ubiquitin protein ligase binding / protein-containing complex binding / positive regulation of gene expression / ubiquitin protein ligase binding / protein-containing complex binding / positive regulation of gene expression /  enzyme binding / identical protein binding / enzyme binding / identical protein binding /  nucleus / nucleus /  plasma membrane / plasma membrane /  cytoplasm cytoplasmSimilarity search - Function | |||||||||||||||
| Biological species |   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat)  Mus musculus (house mouse) Mus musculus (house mouse) | |||||||||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.5 Å cryo EM / Resolution: 3.5 Å | |||||||||||||||
 Authors Authors | Maharana, J. / Sarma, P. / Yadav, M.K. / Chami, M. / Banerjee, R. / Shukla, A.K. | |||||||||||||||
| Funding support |  India, 4items India, 4items
| |||||||||||||||
 Citation Citation |  Journal: Science / Year: 2024 Journal: Science / Year: 2024Title: Molecular insights into atypical modes of β-arrestin interaction with seven transmembrane receptors. Authors: Jagannath Maharana / Fumiya K Sano / Parishmita Sarma / Manish K Yadav / Longhan Duan / Tomasz M Stepniewski / Madhu Chaturvedi / Ashutosh Ranjan / Vinay Singh / Sayantan Saha / Gargi ...Authors: Jagannath Maharana / Fumiya K Sano / Parishmita Sarma / Manish K Yadav / Longhan Duan / Tomasz M Stepniewski / Madhu Chaturvedi / Ashutosh Ranjan / Vinay Singh / Sayantan Saha / Gargi Mahajan / Mohamed Chami / Wataru Shihoya / Jana Selent / Ka Young Chung / Ramanuj Banerjee / Osamu Nureki / Arun K Shukla /      Abstract: β-arrestins (βarrs) are multifunctional proteins involved in signaling and regulation of seven transmembrane receptors (7TMRs), and their interaction is driven primarily by agonist-induced receptor ...β-arrestins (βarrs) are multifunctional proteins involved in signaling and regulation of seven transmembrane receptors (7TMRs), and their interaction is driven primarily by agonist-induced receptor activation and phosphorylation. Here, we present seven cryo-electron microscopy structures of βarrs either in the basal state, activated by the muscarinic receptor subtype 2 (M2R) through its third intracellular loop, or activated by the βarr-biased decoy D6 receptor (D6R). Combined with biochemical, cellular, and biophysical experiments, these structural snapshots allow the visualization of atypical engagement of βarrs with 7TMRs and also reveal a structural transition in the carboxyl terminus of βarr2 from a β strand to an α helix upon activation by D6R. Our study provides previously unanticipated molecular insights into the structural and functional diversity encoded in 7TMR-βarr complexes with direct implications for exploring novel therapeutic avenues. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8j9k.cif.gz 8j9k.cif.gz | 111.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8j9k.ent.gz pdb8j9k.ent.gz | 82.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8j9k.json.gz 8j9k.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/j9/8j9k https://data.pdbj.org/pub/pdb/validation_reports/j9/8j9k ftp://data.pdbj.org/pub/pdb/validation_reports/j9/8j9k ftp://data.pdbj.org/pub/pdb/validation_reports/j9/8j9k | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  36110MC  8go9C  8j8rC  8j8vC  8j8zC  8j97C  8ja3C  8jafC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein |  Arrestin beta 2 / Arrestin beta-2 Arrestin beta 2 / Arrestin beta-2Mass: 44490.906 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Rattus norvegicus (Norway rat) / Gene: Arrb2 / Production host: Rattus norvegicus (Norway rat) / Gene: Arrb2 / Production host:   Escherichia coli (E. coli) / References: UniProt: P29067 Escherichia coli (E. coli) / References: UniProt: P29067 |
|---|---|
| #2: Antibody | Mass: 11356.607 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Mus musculus (house mouse) / Production host: Mus musculus (house mouse) / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| #3: Antibody | Mass: 13636.962 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Mus musculus (house mouse) / Production host: Mus musculus (house mouse) / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | ||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||
| Source (recombinant) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) | ||||||||||||||||||||||||
| Buffer solution | pH: 7.4 | ||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES | ||||||||||||||||||||||||
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Microscopy | Model: TFS GLACIOS |
|---|---|
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2500 nm / Nominal defocus min: 500 nm Bright-field microscopy / Nominal defocus max: 2500 nm / Nominal defocus min: 500 nm |
| Image recording | Electron dose: 55 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| EM software |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Type: NONE | ||||||||||||||||
| Particle selection | Num. of particles selected: 7887274 | ||||||||||||||||
3D reconstruction | Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 506938 / Symmetry type: POINT | ||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: REAL | ||||||||||||||||
| Atomic model building | PDB-ID: 8GOC Accession code: 8GOC / Source name: PDB / Type: experimental model |
 Movie
Movie Controller
Controller



 PDBj
PDBj







