6VXZ
 
 | | SthK P300A cyclic nucleotide-gated potassium channel in the closed state, in complex with cAMP | | Descriptor: | (1R)-2-{[(S)-{[(2S)-2,3-dihydroxypropyl]oxy}(hydroxy)phosphoryl]oxy}-1-[(hexadecanoyloxy)methyl]ethyl (9Z)-octadec-9-enoate, ADENOSINE-3',5'-CYCLIC-MONOPHOSPHATE, SthK | | Authors: | Schmidpeter, P.A.M, Rheinberger, J, Nimigean, C.M. | | Deposit date: | 2020-02-25 | | Release date: | 2020-11-11 | | Last modified: | 2024-03-06 | | Method: | ELECTRON MICROSCOPY (3.42 Å) | | Cite: | Prolyl isomerization controls activation kinetics of a cyclic nucleotide-gated ion channel.
Nat Commun, 11, 2020
|
|
6VY0
 
 | |
8VTA
 
 | | SthK R120A in the presence of PIP2 and cAMP | | Descriptor: | 1,2-DIOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE, ADENOSINE-3',5'-CYCLIC-MONOPHOSPHATE, Transcriptional regulator, ... | | Authors: | Schmidpeter, P.A.M, Thon, O, Nimigean, C.M. | | Deposit date: | 2024-01-26 | | Release date: | 2024-09-25 | | Last modified: | 2024-10-02 | | Method: | ELECTRON MICROSCOPY (3 Å) | | Cite: | PIP2 inhibits pore opening of the cyclic nucleotide-gated channel SthK.
Nat Commun, 15, 2024
|
|
8VTB
 
 | | SthK R120A R124A in the presence of PIP2 and cAMP | | Descriptor: | 1,2-DIOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE, ADENOSINE-3',5'-CYCLIC-MONOPHOSPHATE, Transcriptional regulator, ... | | Authors: | Schmidpeter, P.A.M, Thon, O, Nimigean, C.M. | | Deposit date: | 2024-01-26 | | Release date: | 2024-09-25 | | Last modified: | 2024-10-02 | | Method: | ELECTRON MICROSCOPY (2.5 Å) | | Cite: | PIP2 inhibits pore opening of the cyclic nucleotide-gated channel SthK.
Nat Commun, 15, 2024
|
|
8VT9
 
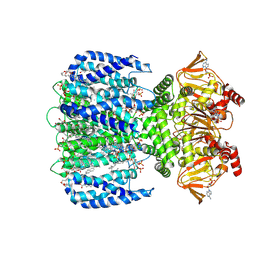 | | WT SthK in the presence of PIP2 and cAMP | | Descriptor: | 1,2-DIOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE, ADENOSINE-3',5'-CYCLIC-MONOPHOSPHATE, Transcriptional regulator, ... | | Authors: | Schmidpeter, P.A.M, Thon, O, Nimigean, C.M. | | Deposit date: | 2024-01-26 | | Release date: | 2024-09-25 | | Last modified: | 2024-10-02 | | Method: | ELECTRON MICROSCOPY (2.9 Å) | | Cite: | PIP2 inhibits pore opening of the cyclic nucleotide-gated channel SthK.
Nat Commun, 15, 2024
|
|
8DE7
 
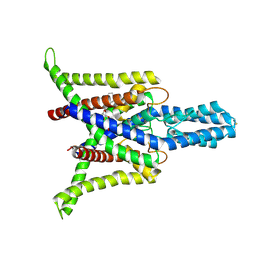 | | Cryo-EM structure of the zebrafish two pore domain K+ channel TREK1 (K2P2.1) in DDM detergent | | Descriptor: | DODECYL-BETA-D-MALTOSIDE, POTASSIUM ION, Potassium channel, ... | | Authors: | Schmidpeter, P.A.M, Nimigean, C.M, Riegelhaupt, P.M. | | Deposit date: | 2022-06-20 | | Release date: | 2023-03-08 | | Last modified: | 2024-10-16 | | Method: | ELECTRON MICROSCOPY (3.27 Å) | | Cite: | Membrane phospholipids control gating of the mechanosensitive potassium leak channel TREK1.
Nat Commun, 14, 2023
|
|
8DE9
 
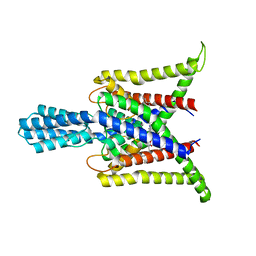 | | Cryo-EM structure of the zebrafish two pore domain K+ channel TREK1 (K2P2.1) in DDM/POPE mixed micelles | | Descriptor: | (1S)-2-{[(2-AMINOETHOXY)(HYDROXY)PHOSPHORYL]OXY}-1-[(PALMITOYLOXY)METHYL]ETHYL STEARATE, POTASSIUM ION, Potassium channel, ... | | Authors: | Schmidpeter, P.A.M, Nimigean, C.M, Riegelhaupt, P.M. | | Deposit date: | 2022-06-20 | | Release date: | 2023-03-08 | | Last modified: | 2024-10-30 | | Method: | ELECTRON MICROSCOPY (3.4 Å) | | Cite: | Membrane phospholipids control gating of the mechanosensitive potassium leak channel TREK1.
Nat Commun, 14, 2023
|
|
8DE8
 
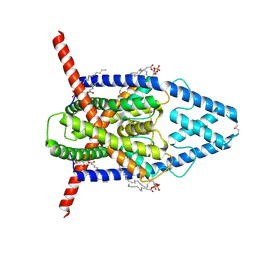 | | Cryo-EM structure of the zebrafish two pore domain K+ channel TREK1 (K2P2.1) in DDM/POPA mixed micelles | | Descriptor: | (2R)-1-(hexadecanoyloxy)-3-(phosphonooxy)propan-2-yl (9Z)-octadec-9-enoate, POTASSIUM ION, Potassium channel, ... | | Authors: | Schmidpeter, P.A.M, Nimigean, C.M, Riegelhaupt, P.M. | | Deposit date: | 2022-06-20 | | Release date: | 2023-03-08 | | Last modified: | 2024-10-09 | | Method: | ELECTRON MICROSCOPY (2.82 Å) | | Cite: | Membrane phospholipids control gating of the mechanosensitive potassium leak channel TREK1.
Nat Commun, 14, 2023
|
|
3ZHH
 
 | | X-ray structure of the full-length beta-lactamase from M.tuberculosis | | Descriptor: | BETA-LACTAMASE, SULFATE ION | | Authors: | Feiler, C, Fisher, A.C, Marrichi, M.J, Wright, L, Schmidpeter, P.A.M, Blankenfeldt, W, Pavelka, M, DeLisa, M.P. | | Deposit date: | 2012-12-21 | | Release date: | 2013-09-25 | | Last modified: | 2023-12-20 | | Method: | X-RAY DIFFRACTION (2.85 Å) | | Cite: | Directed Evolution of Mycobacterium Tuberculosis Beta-Lactamase Reveals Gatekeeper Residue that Regulates Antibiotic Resistance and Catalytic Efficiency.
Plos One, 8, 2013
|
|
