[English] 日本語
 Yorodumi
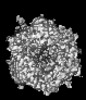
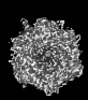
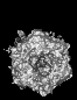
Yorodumi- PDB-9cli: Cryo-EM model derived from localized reconstruction of human aden... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9cli | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM model derived from localized reconstruction of human adenovirus (Ad5)-hexon-FX complex at 3.6A resolution | ||||||
 Components Components |
| ||||||
 Keywords Keywords | VIRUS / Adenovirus / Hexon / Coagulation factor X / Coagulation factor II / Prothrombin / Complex / Interactions | ||||||
| Function / homology |  Function and homology information Function and homology informationT=25 icosahedral viral capsid / coagulation factor Xa / Defective factor IX causes thrombophilia / Defective cofactor function of FVIIIa variant / Defective F9 variant does not activate FX / microtubule-dependent intracellular transport of viral material towards nucleus / Extrinsic Pathway of Fibrin Clot Formation / positive regulation of TOR signaling / Transport of gamma-carboxylated protein precursors from the endoplasmic reticulum to the Golgi apparatus / Gamma-carboxylation of protein precursors ...T=25 icosahedral viral capsid / coagulation factor Xa / Defective factor IX causes thrombophilia / Defective cofactor function of FVIIIa variant / Defective F9 variant does not activate FX / microtubule-dependent intracellular transport of viral material towards nucleus / Extrinsic Pathway of Fibrin Clot Formation / positive regulation of TOR signaling / Transport of gamma-carboxylated protein precursors from the endoplasmic reticulum to the Golgi apparatus / Gamma-carboxylation of protein precursors / Common Pathway of Fibrin Clot Formation / Removal of aminoterminal propeptides from gamma-carboxylated proteins / Intrinsic Pathway of Fibrin Clot Formation / phospholipid binding / Golgi lumen / blood coagulation / viral capsid / host cell / positive regulation of cell migration / endoplasmic reticulum lumen / external side of plasma membrane / serine-type endopeptidase activity / calcium ion binding / symbiont entry into host cell / host cell nucleus / structural molecule activity / proteolysis / extracellular space / extracellular region / plasma membrane Similarity search - Function | ||||||
| Biological species |   Human adenovirus 5 Human adenovirus 5 Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.61 Å | ||||||
 Authors Authors | Reddy, V.S. / Ma, O.X. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structure-derived insights from blood factors binding to the surfaces of different adenoviruses. Authors: Haley E Mudrick / Shao-Chia Lu / Janarjan Bhandari / Mary E Barry / Jack R Hemsath / Felix G M Andres / Olivia X Ma / Michael A Barry / Vijay S Reddy /  Abstract: The tropism of adenoviruses (Ads) is significantly influenced by the binding of various blood factors. To investigate differences in their binding, we conducted cryo-EM analysis on complexes of ...The tropism of adenoviruses (Ads) is significantly influenced by the binding of various blood factors. To investigate differences in their binding, we conducted cryo-EM analysis on complexes of several human adenoviruses with human platelet factor-4 (PF4), coagulation factors FII (Prothrombin), and FX. While we observed EM densities for FII and FX bound to all the species-C adenoviruses examined, no densities were seen for PF4, even though PF4 can co-pellet with various Ads. Similar to FX, the γ-carboxyglutamic acid (Gla) domain of FII binds within the surface cavity of hexon trimers. While FII binds equally to species-C Ads: Ad5, Ad6, and Ad657, FX exhibits significantly better binding to Ad5 and Ad657 compared to Ad6. Although only the FX-Gla domain is observed at high-resolution (3.7 Å), the entire FX is visible at low-resolution bound to Ad5 in three equivalent binding modes consistent with the 3-fold symmetric hexon. Only the Gla and kringle-1 domains of FII are visible on all the species-C adenoviruses, where the rigid FII binds in an upright fashion, in contrast to the flexible and bent FX. These data suggest that differential binding of FII and FX may shield certain species-C adenoviruses differently against immune molecules, thereby modulating their tropism. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9cli.cif.gz 9cli.cif.gz | 559.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9cli.ent.gz pdb9cli.ent.gz | 454 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9cli.json.gz 9cli.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/cl/9cli https://data.pdbj.org/pub/pdb/validation_reports/cl/9cli ftp://data.pdbj.org/pub/pdb/validation_reports/cl/9cli ftp://data.pdbj.org/pub/pdb/validation_reports/cl/9cli | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 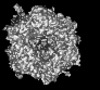 45675MC  9clnC  9clsC  9cm2C  9cm9C  9cmoC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 108107.617 Da / Num. of mol.: 3 / Source method: isolated from a natural source / Details: Adenovirus hexon protein / Source: (natural)   Human adenovirus 5 / References: UniProt: P04133 Human adenovirus 5 / References: UniProt: P04133#2: Protein | | Mass: 55286.738 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: P00742, coagulation factor Xa Homo sapiens (human) / References: UniProt: P00742, coagulation factor Xa#3: Chemical | ChemComp-CA / Has ligand of interest | N | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.4 MDa / Experimental value: NO | ||||||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||||||
| Details of virus | Empty: NO / Enveloped: NO / Isolate: SPECIES / Type: VIRION | ||||||||||||||||||||||||||||
| Natural host | Organism: Human adenovirus 5 | ||||||||||||||||||||||||||||
| Virus shell |
| ||||||||||||||||||||||||||||
| Buffer solution | pH: 7.2 | ||||||||||||||||||||||||||||
| Buffer component | Conc.: 20 mM / Name: Hepes | ||||||||||||||||||||||||||||
| Specimen | Conc.: 2 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||||||
| Specimen support | Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 81000 X / Calibrated magnification: 81000 X / Nominal defocus max: 4000 nm / Nominal defocus min: 2500 nm / Calibrated defocus min: 1000 nm / Calibrated defocus max: 5000 nm / Cs: 2.7 mm / C2 aperture diameter: 100 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 81 e/Å2 / Film or detector model: GATAN K3 BIOCONTINUUM (6k x 4k) / Num. of grids imaged: 3 / Num. of real images: 10000 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: NONE | ||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 59000 | ||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.61 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 26000 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT | ||||||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 6B1T Accession code: 6B1T / Source name: PDB / Type: experimental model |
 Movie
Movie Controller
Controller








 PDBj
PDBj













