[English] 日本語
 Yorodumi
Yorodumi- PDB-7oqu: Ternary complex of 14-3-3 sigma, Amot-p130 phosphopeptide, and WQ180 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7oqu | ||||||
|---|---|---|---|---|---|---|---|
| Title | Ternary complex of 14-3-3 sigma, Amot-p130 phosphopeptide, and WQ180 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | SIGNALING PROTEIN / protein-peptide complex small-molecule | ||||||
| Function / homology |  Function and homology information Function and homology informationestablishment of cell polarity involved in ameboidal cell migration / cell migration involved in gastrulation / blood vessel endothelial cell migration / Regulation of CDH11 function / positive regulation of embryonic development / angiostatin binding / regulation of modification of postsynaptic actin cytoskeleton / hippo signaling / gastrulation with mouth forming second / negative regulation of vascular permeability ...establishment of cell polarity involved in ameboidal cell migration / cell migration involved in gastrulation / blood vessel endothelial cell migration / Regulation of CDH11 function / positive regulation of embryonic development / angiostatin binding / regulation of modification of postsynaptic actin cytoskeleton / hippo signaling / gastrulation with mouth forming second / negative regulation of vascular permeability / cell-cell junction assembly / Signaling by Hippo / regulation of small GTPase mediated signal transduction / regulation of epidermal cell division / protein kinase C inhibitor activity / positive regulation of epidermal cell differentiation / keratinocyte development / keratinization / regulation of cell-cell adhesion / cAMP/PKA signal transduction / Regulation of localization of FOXO transcription factors / keratinocyte proliferation / phosphoserine residue binding / endocytic vesicle / positive regulation of blood vessel endothelial cell migration / positive regulation of cell size / Activation of BAD and translocation to mitochondria / bicellular tight junction / negative regulation of keratinocyte proliferation / establishment of skin barrier / negative regulation of protein localization to plasma membrane / vasculogenesis / Chk1/Chk2(Cds1) mediated inactivation of Cyclin B:Cdk1 complex / SARS-CoV-2 targets host intracellular signalling and regulatory pathways / negative regulation of protein kinase activity / negative regulation of stem cell proliferation / stress fiber / SARS-CoV-1 targets host intracellular signalling and regulatory pathways / RHO GTPases activate PKNs / positive regulation of stress fiber assembly / positive regulation of protein localization / ruffle / positive regulation of cell adhesion / protein sequestering activity / negative regulation of innate immune response / protein export from nucleus / regulation of cell migration / negative regulation of angiogenesis / TP53 Regulates Transcription of Genes Involved in G2 Cell Cycle Arrest / release of cytochrome c from mitochondria / positive regulation of protein export from nucleus / stem cell proliferation / Translocation of SLC2A4 (GLUT4) to the plasma membrane / actin filament / TP53 Regulates Metabolic Genes / chemotaxis / intrinsic apoptotic signaling pathway in response to DNA damage / intracellular protein localization / lamellipodium / signaling receptor activity / regulation of protein localization / actin cytoskeleton organization / positive regulation of cell growth / cytoplasmic vesicle / angiogenesis / in utero embryonic development / regulation of cell cycle / postsynaptic density / cadherin binding / external side of plasma membrane / protein kinase binding / glutamatergic synapse / cell surface / negative regulation of transcription by RNA polymerase II / signal transduction / extracellular space / extracellular exosome / identical protein binding / nucleus / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.4 Å MOLECULAR REPLACEMENT / Resolution: 1.4 Å | ||||||
 Authors Authors | Centorrino, F. / Ottmann, C. | ||||||
| Funding support | European Union, 1items
| ||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: A crystallography-based study of fragment extensions into the 14-3-3 binding groove Authors: Centorrino, F. / Wu, Q. / Cossar, P. / Brunsveld, L. / Ottmann, C. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7oqu.cif.gz 7oqu.cif.gz | 130.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7oqu.ent.gz pdb7oqu.ent.gz | 81.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7oqu.json.gz 7oqu.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7oqu_validation.pdf.gz 7oqu_validation.pdf.gz | 790.6 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7oqu_full_validation.pdf.gz 7oqu_full_validation.pdf.gz | 791.4 KB | Display | |
| Data in XML |  7oqu_validation.xml.gz 7oqu_validation.xml.gz | 13.3 KB | Display | |
| Data in CIF |  7oqu_validation.cif.gz 7oqu_validation.cif.gz | 19.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/oq/7oqu https://data.pdbj.org/pub/pdb/validation_reports/oq/7oqu ftp://data.pdbj.org/pub/pdb/validation_reports/oq/7oqu ftp://data.pdbj.org/pub/pdb/validation_reports/oq/7oqu | HTTPS FTP |
-Related structure data
| Related structure data |  7opwC  7oq7C  7oq8C 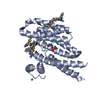 7oq9C  7oqaC  7oqgC  7oqjC  7oqsC  7oqwC  7or3C  7or5C  7or7C  7or8C  7orgC  7orhC  7orsC  7ortC  4jc3S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein / Protein/peptide , 2 types, 2 molecules AP
| #1: Protein | Mass: 28226.518 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SFN, HME1 / Production host: Homo sapiens (human) / Gene: SFN, HME1 / Production host:  |
|---|---|
| #2: Protein/peptide | Mass: 1626.817 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.)  Homo sapiens (human) / References: UniProt: Q4VCS5 Homo sapiens (human) / References: UniProt: Q4VCS5 |
-Non-polymers , 4 types, 238 molecules 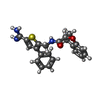






| #3: Chemical | ChemComp-0BL / ( | ||
|---|---|---|---|
| #4: Chemical | ChemComp-CL / | ||
| #5: Chemical | | #6: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.41 Å3/Da / Density % sol: 48.94 % |
|---|---|
| Crystal grow | Temperature: 277.15 K / Method: vapor diffusion, hanging drop Details: 0.095 M Hepes pH7.5, 26%PEG 400, 0.19 M CaCl2, and 5 % Glycerol |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID30B / Wavelength: 0.97626 Å / Beamline: ID30B / Wavelength: 0.97626 Å |
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: Feb 5, 2021 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97626 Å / Relative weight: 1 |
| Reflection | Resolution: 1.4→66.45 Å / Num. obs: 57063 / % possible obs: 100 % / Redundancy: 12.9 % / Biso Wilson estimate: 15.31 Å2 / CC1/2: 0.998 / Rmerge(I) obs: 0.107 / Net I/σ(I): 12.1 |
| Reflection shell | Resolution: 1.4→1.42 Å / Redundancy: 13 % / Rmerge(I) obs: 1.672 / Mean I/σ(I) obs: 1.9 / Num. unique obs: 2810 / CC1/2: 0.799 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4JC3 Resolution: 1.4→66.45 Å / SU ML: 0.1291 / Cross valid method: FREE R-VALUE / σ(F): 1.33 / Phase error: 17.9852 / Stereochemistry target values: GeoStd + Monomer Library
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 22.92 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.4→66.45 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj














