[English] 日本語
 Yorodumi
Yorodumi- PDB-7or3: Ternary complex of 14-3-3 sigma, NotchpS1917 phosphopeptide, and WQ136 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7or3 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Ternary complex of 14-3-3 sigma, NotchpS1917 phosphopeptide, and WQ136 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | SIGNALING PROTEIN / protein-peptide complex small-molecule | ||||||
| Function / homology |  Function and homology information Function and homology informationmorphogenesis of a branching structure / negative regulation of endothelial cell differentiation / Defective LFNG causes SCDO3 / Pre-NOTCH Processing in the Endoplasmic Reticulum / positive regulation of transcription of Notch receptor target / positive regulation of smooth muscle cell differentiation / negative regulation of cell adhesion molecule production / Pre-NOTCH Processing in Golgi / NOTCH4 Activation and Transmission of Signal to the Nucleus / endothelial cell morphogenesis ...morphogenesis of a branching structure / negative regulation of endothelial cell differentiation / Defective LFNG causes SCDO3 / Pre-NOTCH Processing in the Endoplasmic Reticulum / positive regulation of transcription of Notch receptor target / positive regulation of smooth muscle cell differentiation / negative regulation of cell adhesion molecule production / Pre-NOTCH Processing in Golgi / NOTCH4 Activation and Transmission of Signal to the Nucleus / endothelial cell morphogenesis / luteolysis / cell fate determination / negative regulation of cell-cell adhesion mediated by cadherin / mammary gland development / vasculature development / Notch binding / NOTCH4 Intracellular Domain Regulates Transcription / branching involved in blood vessel morphogenesis / Notch-HLH transcription pathway / regulation of epidermal cell division / protein kinase C inhibitor activity / positive regulation of epidermal cell differentiation / keratinocyte development / keratinization / regulation of cell-cell adhesion / hemopoiesis / cAMP/PKA signal transduction / Regulation of localization of FOXO transcription factors / keratinocyte proliferation / epithelial to mesenchymal transition / negative regulation of cell differentiation / Activation of BAD and translocation to mitochondria / phosphoserine residue binding / negative regulation of keratinocyte proliferation / establishment of skin barrier / negative regulation of protein localization to plasma membrane / Chk1/Chk2(Cds1) mediated inactivation of Cyclin B:Cdk1 complex / SARS-CoV-2 targets host intracellular signalling and regulatory pathways / negative regulation of protein kinase activity / negative regulation of stem cell proliferation / cis-regulatory region sequence-specific DNA binding / RHO GTPases activate PKNs / SARS-CoV-1 targets host intracellular signalling and regulatory pathways / positive regulation of protein localization / positive regulation of cell adhesion / protein sequestering activity / negative regulation of innate immune response / TP53 Regulates Transcription of Genes Involved in G2 Cell Cycle Arrest / protein export from nucleus / release of cytochrome c from mitochondria / stem cell proliferation / positive regulation of protein export from nucleus / TP53 Regulates Metabolic Genes / Translocation of SLC2A4 (GLUT4) to the plasma membrane / Negative regulation of NOTCH4 signaling / Pre-NOTCH Transcription and Translation / intrinsic apoptotic signaling pathway in response to DNA damage / intracellular protein localization / signaling receptor activity / regulation of protein localization / positive regulation of cell growth / DNA-binding transcription activator activity, RNA polymerase II-specific / cell differentiation / regulation of cell cycle / cadherin binding / Golgi membrane / calcium ion binding / protein kinase binding / endoplasmic reticulum membrane / positive regulation of DNA-templated transcription / cell surface / negative regulation of transcription by RNA polymerase II / signal transduction / positive regulation of transcription by RNA polymerase II / extracellular space / extracellular exosome / extracellular region / nucleoplasm / identical protein binding / nucleus / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å MOLECULAR REPLACEMENT / Resolution: 1.8 Å | ||||||
 Authors Authors | Centorrino, F. / Wu, Q. / Ottmann, C. | ||||||
| Funding support | European Union, 1items
| ||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: A crystallography-based study of fragment extensions into the 14-3-3 binding groove Authors: Centorrino, F. / Wu, Q. / Cossar, P. / Brunsveld, L. / Ottmann, C. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7or3.cif.gz 7or3.cif.gz | 134.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7or3.ent.gz pdb7or3.ent.gz | 85.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7or3.json.gz 7or3.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7or3_validation.pdf.gz 7or3_validation.pdf.gz | 1.3 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7or3_full_validation.pdf.gz 7or3_full_validation.pdf.gz | 1.3 MB | Display | |
| Data in XML |  7or3_validation.xml.gz 7or3_validation.xml.gz | 14.7 KB | Display | |
| Data in CIF |  7or3_validation.cif.gz 7or3_validation.cif.gz | 21.9 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/or/7or3 https://data.pdbj.org/pub/pdb/validation_reports/or/7or3 ftp://data.pdbj.org/pub/pdb/validation_reports/or/7or3 ftp://data.pdbj.org/pub/pdb/validation_reports/or/7or3 | HTTPS FTP |
-Related structure data
| Related structure data |  7opwC  7oq7C  7oq8C  7oq9C  7oqaC  7oqgC  7oqjC  7oqsC  7oquC  7oqwC  7or5C  7or7C  7or8C  7orgC  7orhC  7orsC  7ortC  4jc3S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein / Protein/peptide , 2 types, 2 molecules AB
| #1: Protein | Mass: 28226.518 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SFN, HME1 / Production host: Homo sapiens (human) / Gene: SFN, HME1 / Production host:  |
|---|---|
| #2: Protein/peptide | Mass: 1334.450 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.)  Homo sapiens (human) / References: UniProt: Q99466 Homo sapiens (human) / References: UniProt: Q99466 |
-Non-polymers , 4 types, 301 molecules 

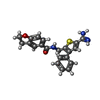




| #3: Chemical | ChemComp-CL / | ||
|---|---|---|---|
| #4: Chemical | ChemComp-MG / | ||
| #5: Chemical | | #6: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.44 Å3/Da / Density % sol: 49.49 % |
|---|---|
| Crystal grow | Temperature: 277.15 K / Method: vapor diffusion, sitting drop Details: 0.095 M Hepes pH7.7, 26%PEG 400, 0.19 M CaCl2, and 5 % Glycerol |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source: SEALED TUBE / Type: RIGAKU MICROMAX-003 / Wavelength: 1.54187 Å |
| Detector | Type: DECTRIS PILATUS 200K / Detector: PIXEL / Date: Feb 16, 2021 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.54187 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→66.26 Å / Num. obs: 23871 / % possible obs: 88.7 % / Redundancy: 3 % / Biso Wilson estimate: 8.99 Å2 / CC1/2: 0.948 / Rmerge(I) obs: 0.074 / Net I/σ(I): 9.5 |
| Reflection shell | Resolution: 1.8→1.83 Å / Redundancy: 2.7 % / Rmerge(I) obs: 0.177 / Mean I/σ(I) obs: 2.4 / Num. unique obs: 1135 / CC1/2: 0.967 / % possible all: 83.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4jc3 Resolution: 1.8→66.26 Å / SU ML: 0.2135 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 22.9309 / Stereochemistry target values: GeoStd + Monomer Library
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 12.51 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.8→66.26 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj




















