[English] 日本語
 Yorodumi
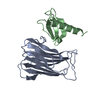
Yorodumi- PDB-7dyu: Human JMJD5 in complex with MN and 5-((4-phenylbutyl)amino)pyridi... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7dyu | ||||||
|---|---|---|---|---|---|---|---|
| Title | Human JMJD5 in complex with MN and 5-((4-phenylbutyl)amino)pyridine-2,4-dicarboxylic acid. | ||||||
 Components Components | Bifunctional peptidase and arginyl-hydroxylase JMJD5 | ||||||
 Keywords Keywords | OXIDOREDUCTASE / JmjC domain-containing protein 5 / JMJD5 / dioxygenase | ||||||
| Function / homology |  Function and homology information Function and homology information[protein]-arginine 3-hydroxylase / peptidyl-arginine 3-dioxygenase activity / histone H3K36 demethylase activity / Hydrolases; Acting on peptide bonds (peptidases) / Protein hydroxylation / aminopeptidase activity / regulation of signal transduction by p53 class mediator / circadian regulation of gene expression / protein destabilization / G2/M transition of mitotic cell cycle ...[protein]-arginine 3-hydroxylase / peptidyl-arginine 3-dioxygenase activity / histone H3K36 demethylase activity / Hydrolases; Acting on peptide bonds (peptidases) / Protein hydroxylation / aminopeptidase activity / regulation of signal transduction by p53 class mediator / circadian regulation of gene expression / protein destabilization / G2/M transition of mitotic cell cycle / p53 binding / chromosome / fibroblast proliferation / endopeptidase activity / in utero embryonic development / negative regulation of DNA-templated transcription / chromatin binding / positive regulation of DNA-templated transcription / proteolysis / nucleoplasm / metal ion binding / nucleus / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.72 Å MOLECULAR REPLACEMENT / Resolution: 1.72 Å | ||||||
 Authors Authors | Nakashima, Y. / Brewitz, L. / Schofield, C.J. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2023 Journal: J.Med.Chem. / Year: 2023Title: 5-Substituted Pyridine-2,4-dicarboxylate Derivatives Have Potential for Selective Inhibition of Human Jumonji-C Domain-Containing Protein 5. Authors: Brewitz, L. / Nakashima, Y. / Piasecka, S.K. / Salah, E. / Fletcher, S.C. / Tumber, A. / Corner, T.P. / Kennedy, T.J. / Fiorini, G. / Thalhammer, A. / Christensen, K.E. / Coleman, M.L. / Schofield, C.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7dyu.cif.gz 7dyu.cif.gz | 193.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7dyu.ent.gz pdb7dyu.ent.gz | 128.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7dyu.json.gz 7dyu.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dy/7dyu https://data.pdbj.org/pub/pdb/validation_reports/dy/7dyu ftp://data.pdbj.org/pub/pdb/validation_reports/dy/7dyu ftp://data.pdbj.org/pub/pdb/validation_reports/dy/7dyu | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7dytC  7dyvC  7dywC  7dyxC  6f4pS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 27261.873 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: KDM8, JMJD5 / Production host: Homo sapiens (human) / Gene: KDM8, JMJD5 / Production host:  References: UniProt: Q8N371, Oxidoreductases; Acting on paired donors, with incorporation or reduction of molecular oxygen; With 2-oxoglutarate as one donor, and incorporation of one atom of oxygen ...References: UniProt: Q8N371, Oxidoreductases; Acting on paired donors, with incorporation or reduction of molecular oxygen; With 2-oxoglutarate as one donor, and incorporation of one atom of oxygen into each donor, Hydrolases; Acting on peptide bonds (peptidases) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| #2: Chemical | | #3: Chemical | ChemComp-MN / | #4: Chemical | ChemComp-HR9 / | #5: Water | ChemComp-HOH / | Has ligand of interest | Y | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.36 Å3/Da / Density % sol: 47.85 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 7.5 Details: 100mM HEPES sodium, 200mM magnesium chloride hexahydrate, 25% w/v PEG 3350, 1mM manganese chloride |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I24 / Wavelength: 0.96862 Å / Beamline: I24 / Wavelength: 0.96862 Å |
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: Nov 28, 2019 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.96862 Å / Relative weight: 1 |
| Reflection | Resolution: 1.72→49.86 Å / Num. obs: 38768 / % possible obs: 94.2 % / Redundancy: 12.4 % / Biso Wilson estimate: 22.73 Å2 / CC1/2: 0.996 / Rmerge(I) obs: 0.155 / Net I/σ(I): 11.9 |
| Reflection shell | Resolution: 1.72→1.85 Å / Redundancy: 8.4 % / Rmerge(I) obs: 1.805 / Mean I/σ(I) obs: 1.2 / Num. unique obs: 1040 / CC1/2: 0.351 / % possible all: 58.5 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6F4P Resolution: 1.72→49.86 Å / SU ML: 0.1264 / Cross valid method: FREE R-VALUE / σ(F): 1.92 / Phase error: 22.1821 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 30.67 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.72→49.86 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj







