[English] 日本語
 Yorodumi
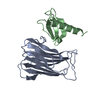
Yorodumi- PDB-6i9n: JmjC domain-containing protein 5 (JMJD5) in complex with Mn and L... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6i9n | ||||||
|---|---|---|---|---|---|---|---|
| Title | JmjC domain-containing protein 5 (JMJD5) in complex with Mn and L-2-hydroxyglutarate | ||||||
 Components Components | JmjC domain-containing protein 5 | ||||||
 Keywords Keywords | OXIDOREDUCTASE / NON-HEME / IRON / 2-OXOGLUTARATE / DIOXYGENASE / JMJC / JMJC DOMAIN / Lysine-specific demethylase 8 / JmjC domain-containing protein 5 / Arginyl C-3 Hydroxylase / JMJD5 / KDM8 / OXYGENASE / HYPOXIA / DNA-BINDING / METAL-BINDING / TRANSLATION / DSBH / FACIAL TRIAD / CYTOPLASM / JMJC HYDROXYLASE / JMJC DEMETHYLASE / KDMS / POST-TRANSLATIONAL MODIFICATIONS / PTM / BETA-HYDROXYLATION / HYDROXYLATION / ARGININE HYDROXYLATION / RCC1 domain-containing protein 1 / RCCD1 / Regulator of chromosome condensation / 40S ribosomal protein S6 / RPS6 / RIBOSOME BIOGENESIS / TRANSCRIPTION / EPIGENETIC REGULATION / SIGNALING / DEVELOPMENT / CELL STRUCTURE / TRANSCRIPTION ACTIVATOR/INHIBITOR / PHOSPHORYLATION / CANCER / POLYMORPHISM | ||||||
| Function / homology |  Function and homology information Function and homology information[protein]-arginine 3-hydroxylase / peptidyl-arginine 3-dioxygenase activity / histone H3K36 demethylase activity / Hydrolases; Acting on peptide bonds (peptidases) / Protein hydroxylation / aminopeptidase activity / regulation of signal transduction by p53 class mediator / circadian regulation of gene expression / protein destabilization / G2/M transition of mitotic cell cycle ...[protein]-arginine 3-hydroxylase / peptidyl-arginine 3-dioxygenase activity / histone H3K36 demethylase activity / Hydrolases; Acting on peptide bonds (peptidases) / Protein hydroxylation / aminopeptidase activity / regulation of signal transduction by p53 class mediator / circadian regulation of gene expression / protein destabilization / G2/M transition of mitotic cell cycle / p53 binding / chromosome / fibroblast proliferation / endopeptidase activity / in utero embryonic development / negative regulation of DNA-templated transcription / chromatin binding / positive regulation of DNA-templated transcription / proteolysis / nucleoplasm / metal ion binding / nucleus / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.361 Å MOLECULAR REPLACEMENT / Resolution: 1.361 Å | ||||||
 Authors Authors | Chowdhury, R. / Islam, M.S. / Schofield, C.J. | ||||||
 Citation Citation |  Journal: Sci Rep / Year: 2022 Journal: Sci Rep / Year: 2022Title: Structural analysis of the 2-oxoglutarate binding site of the circadian rhythm linked oxygenase JMJD5. Authors: Islam, M.S. / Markoulides, M. / Chowdhury, R. / Schofield, C.J. #1:  Journal: Nat Commun / Year: 2018 Journal: Nat Commun / Year: 2018Title: JMJD5 is a human arginyl C-3 hydroxylase. Authors: Wilkins, S.E. / Islam, M.S. / Gannon, J.M. / Markolovic, S. / Hopkinson, R.J. / Ge, W. / Schofield, C.J. / Chowdhury, R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6i9n.cif.gz 6i9n.cif.gz | 115.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6i9n.ent.gz pdb6i9n.ent.gz | 89.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6i9n.json.gz 6i9n.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/i9/6i9n https://data.pdbj.org/pub/pdb/validation_reports/i9/6i9n ftp://data.pdbj.org/pub/pdb/validation_reports/i9/6i9n ftp://data.pdbj.org/pub/pdb/validation_reports/i9/6i9n | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6i9lC  6i9mC  7uq3C  4gjzS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 29733.547 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: CATALYTIC DOMAIN (RESIDUES 183-416) / Source: (gene. exp.)  Homo sapiens (human) / Gene: KDM8, JMJD5 / Production host: Homo sapiens (human) / Gene: KDM8, JMJD5 / Production host:  References: UniProt: Q8N371, [histone H3]-dimethyl-L-lysine36 demethylase, Hydrolases; Acting on peptide bonds (peptidases) |
|---|
-Non-polymers , 5 types, 299 molecules 








| #2: Chemical | ChemComp-MN / |
|---|---|
| #3: Chemical | ChemComp-S2G / ( |
| #4: Chemical | ChemComp-TRS / |
| #5: Chemical | ChemComp-GOL / |
| #6: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.33 Å3/Da / Density % sol: 47.33 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: 0.1 M Bis-Tris pH 6.5, 15.0 % PEG3350, 0.002 M MnCl2 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04-1 / Wavelength: 0.91742 Å / Beamline: I04-1 / Wavelength: 0.91742 Å |
| Detector | Type: DECTRIS PILATUS 2M / Detector: PIXEL / Date: Mar 5, 2015 / Details: MIRRORS |
| Radiation | Monochromator: SI 111 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.91742 Å / Relative weight: 1 |
| Reflection | Resolution: 1.34→41.808 Å / Num. obs: 53414 / % possible obs: 98.8 % / Redundancy: 9.6 % / Biso Wilson estimate: 14.5 Å2 / CC1/2: 0.997 / Rmerge(I) obs: 0.081 / Rpim(I) all: 0.028 / Rrim(I) all: 0.086 / Net I/σ(I): 24.125 |
| Reflection shell | Resolution: 1.34→1.41 Å / Redundancy: 8.9 % / Rmerge(I) obs: 0.529 / Mean I/σ(I) obs: 4.2 / Num. unique obs: 5302 / CC1/2: 0.934 / Rpim(I) all: 0.186 / Rrim(I) all: 0.561 / % possible all: 99.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4GJZ Resolution: 1.361→41.808 Å / SU ML: 0.1 / Cross valid method: THROUGHOUT / σ(F): 1.34 / Phase error: 15.56
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Bsol: 53.5 Å2 / ksol: 0.43 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 22 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.361→41.808 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj





