Entry Database : PDB / ID : 7d6fTitle The crystal structure of ARMS-PBM/MAGI2-PDZ4 Kinase D-interacting substrate of 220 kDa Membrane-associated guanylate kinase, WW and PDZ domain-containing protein 2 Keywords / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Mus musculus (house mouse)Rattus norvegicus (Norway rat)Method / / / Resolution : 3.001 Å Authors Ye, J. / Zhang, Y. / Zhong, Z. / Wang, C. Journal : Neurochem.Int. / Year : 2021Title : Crystal structure of the PDZ4 domain of MAGI2 in complex with PBM of ARMS reveals a canonical PDZ recognition mode.Authors : Zhang, Y. / Zhong, Z. / Ye, J. / Wang, C. History Deposition Sep 30, 2020 Deposition site / Processing site Revision 1.0 Nov 4, 2020 Provider / Type Revision 2.0 Sep 1, 2021 Group Advisory / Atomic model ... Advisory / Atomic model / Author supporting evidence / Data collection / Database references / Derived calculations / Non-polymer description / Refinement description / Structure summary Category atom_site / atom_site_anisotrop ... atom_site / atom_site_anisotrop / audit_author / chem_comp / citation_author / database_2 / entity / pdbx_entity_instance_feature / pdbx_entity_nonpoly / pdbx_entry_details / pdbx_nonpoly_scheme / pdbx_poly_seq_scheme / pdbx_refine_tls / pdbx_refine_tls_group / pdbx_struct_assembly_gen / pdbx_struct_assembly_prop / pdbx_struct_sheet_hbond / pdbx_validate_rmsd_angle / pdbx_validate_symm_contact / pdbx_validate_torsion / refine / refine_hist / refine_ls_shell / reflns / software / struct_asym / struct_conf / struct_sheet / struct_sheet_order / struct_sheet_range Item _audit_author.name / _chem_comp.formula ... _audit_author.name / _chem_comp.formula / _chem_comp.formula_weight / _chem_comp.id / _chem_comp.mon_nstd_flag / _chem_comp.name / _chem_comp.pdbx_synonyms / _chem_comp.type / _citation_author.name / _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_poly_seq_scheme.auth_mon_id / _pdbx_refine_tls.L[1][1] / _pdbx_refine_tls.L[1][2] / _pdbx_refine_tls.L[1][3] / _pdbx_refine_tls.L[2][2] / _pdbx_refine_tls.L[2][3] / _pdbx_refine_tls.L[3][3] / _pdbx_refine_tls.S[1][1] / _pdbx_refine_tls.S[1][2] / _pdbx_refine_tls.S[1][3] / _pdbx_refine_tls.S[2][1] / _pdbx_refine_tls.S[2][2] / _pdbx_refine_tls.S[2][3] / _pdbx_refine_tls.S[3][1] / _pdbx_refine_tls.S[3][2] / _pdbx_refine_tls.S[3][3] / _pdbx_refine_tls.T[1][1] / _pdbx_refine_tls.T[1][2] / _pdbx_refine_tls.T[1][3] / _pdbx_refine_tls.T[2][2] / _pdbx_refine_tls.T[2][3] / _pdbx_refine_tls.T[3][3] / _pdbx_refine_tls.origin_x / _pdbx_refine_tls.origin_y / _pdbx_refine_tls.origin_z / _pdbx_struct_assembly_gen.asym_id_list / _pdbx_struct_assembly_prop.value / _refine.B_iso_max / _refine.B_iso_mean / _refine.B_iso_min / _refine.ls_R_factor_R_free / _refine.ls_R_factor_R_work / _refine.ls_R_factor_obs / _refine.ls_d_res_high / _refine.ls_number_reflns_R_free / _refine.ls_number_reflns_R_work / _refine.ls_number_reflns_obs / _refine.ls_percent_reflns_R_free / _refine.ls_percent_reflns_obs / _refine.overall_SU_ML / _refine.pdbx_ls_sigma_F / _refine.pdbx_overall_phase_error / _refine.pdbx_starting_model / _refine_hist.d_res_high / _refine_hist.number_atoms_solvent / _refine_hist.number_atoms_total / _refine_hist.pdbx_B_iso_mean_ligand / _refine_hist.pdbx_B_iso_mean_solvent / _refine_hist.pdbx_number_atoms_ligand / _refine_ls_shell.R_factor_R_free / _refine_ls_shell.R_factor_R_work / _refine_ls_shell.d_res_high / _refine_ls_shell.d_res_low / _refine_ls_shell.number_reflns_R_free / _refine_ls_shell.number_reflns_R_work / _refine_ls_shell.percent_reflns_obs / _reflns.B_iso_Wilson_estimate / _reflns.d_resolution_high / _reflns.pdbx_number_measured_all / _software.version / _struct_conf.end_auth_comp_id / _struct_conf.end_auth_seq_id / _struct_conf.end_label_comp_id / _struct_conf.end_label_seq_id / _struct_conf.pdbx_PDB_helix_length / _struct_sheet.number_strands Description Details Provider / Type Revision 2.1 Nov 17, 2021 Group / Category / citation_authorItem _citation.country / _citation.journal_abbrev ... _citation.country / _citation.journal_abbrev / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year / _citation_author.name Revision 2.2 Nov 29, 2023 Group / Refinement descriptionCategory / chem_comp_bond / pdbx_initial_refinement_model
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information

 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.001 Å
MOLECULAR REPLACEMENT / Resolution: 3.001 Å  Authors
Authors Citation
Citation Journal: Neurochem.Int. / Year: 2021
Journal: Neurochem.Int. / Year: 2021 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 7d6f.cif.gz
7d6f.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb7d6f.ent.gz
pdb7d6f.ent.gz PDB format
PDB format 7d6f.json.gz
7d6f.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/d6/7d6f
https://data.pdbj.org/pub/pdb/validation_reports/d6/7d6f ftp://data.pdbj.org/pub/pdb/validation_reports/d6/7d6f
ftp://data.pdbj.org/pub/pdb/validation_reports/d6/7d6f
 Links
Links Assembly
Assembly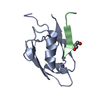
 Components
Components



 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  SSRF
SSRF  / Beamline: BL19U1 / Wavelength: 0.97776 Å
/ Beamline: BL19U1 / Wavelength: 0.97776 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller












 PDBj
PDBj





