| Entry | Database: PDB / ID: 5cax
|
|---|
| Title | CRYSTAL STRUCTURE OF METHANOSARCINA ACETIVORANS METHANOREDOXIN |
|---|
 Components Components | Glutaredoxin |
|---|
 Keywords Keywords | OXIDOREDUCTASE / METHANOGENESIS / OXIDATIVE STRESS / COENZYME M |
|---|
| Function / homology |  Function and homology information Function and homology information
Glutaredoxin active site / Glutaredoxin active site. / Glutaredoxin / Glutaredoxin / Glutaredoxin domain profile. / Glutaredoxin / Glutaredoxin / Thioredoxin-like superfamily / 3-Layer(aba) Sandwich / Alpha BetaSimilarity search - Domain/homology |
|---|
| Biological species |  Methanosarcina acetivorans (archaea) Methanosarcina acetivorans (archaea) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.451 Å MOLECULAR REPLACEMENT / Resolution: 2.451 Å |
|---|
 Authors Authors | Yennawar, N.H. / Yennawar, H.P. / Ferry, G.J. |
|---|
| Funding support |  United States, 1items United States, 1items | Organization | Grant number | Country |
|---|
| Department of Energy (DOE, United States) | DE-FG02-95ER20198 MOD16 |  United States United States |
|
|---|
 Citation Citation |  Journal: Biochemistry / Year: 2016 Journal: Biochemistry / Year: 2016
Title: Structural and Biochemical Characterizations of Methanoredoxin from Methanosarcina acetivorans, a Glutaredoxin-Like Enzyme with Coenzyme M-Dependent Protein Disulfide Reductase Activity.
Authors: Yenugudhati, D. / Prakash, D. / Kumar, A.K. / Kumar, R.S. / Yennawar, N.H. / Yennawar, H.P. / Ferry, J.G. |
|---|
| History | | Deposition | Jun 30, 2015 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | Feb 24, 2016 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Sep 27, 2017 | Group: Author supporting evidence / Derived calculations / Category: pdbx_audit_support / pdbx_struct_oper_list
Item: _pdbx_audit_support.funding_organization / _pdbx_struct_oper_list.symmetry_operation |
|---|
| Revision 1.2 | Dec 4, 2019 | Group: Author supporting evidence / Category: pdbx_audit_support / Item: _pdbx_audit_support.funding_organization |
|---|
| Revision 1.3 | Sep 27, 2023 | Group: Data collection / Database references ...Data collection / Database references / Derived calculations / Refinement description
Category: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model / pdbx_struct_conn_angle / struct_conn
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession ..._database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_conn_angle.ptnr1_auth_asym_id / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_asym_id / _pdbx_struct_conn_angle.ptnr1_label_atom_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr1_symmetry / _pdbx_struct_conn_angle.ptnr2_auth_asym_id / _pdbx_struct_conn_angle.ptnr2_auth_seq_id / _pdbx_struct_conn_angle.ptnr2_label_asym_id / _pdbx_struct_conn_angle.ptnr3_auth_asym_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_asym_id / _pdbx_struct_conn_angle.ptnr3_label_atom_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.ptnr3_symmetry / _pdbx_struct_conn_angle.value / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr1_symmetry / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_symmetry |
|---|
|
|---|
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Methanosarcina acetivorans (archaea)
Methanosarcina acetivorans (archaea) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.451 Å
MOLECULAR REPLACEMENT / Resolution: 2.451 Å  Authors
Authors United States, 1items
United States, 1items  Citation
Citation Journal: Biochemistry / Year: 2016
Journal: Biochemistry / Year: 2016 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5cax.cif.gz
5cax.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5cax.ent.gz
pdb5cax.ent.gz PDB format
PDB format 5cax.json.gz
5cax.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ca/5cax
https://data.pdbj.org/pub/pdb/validation_reports/ca/5cax ftp://data.pdbj.org/pub/pdb/validation_reports/ca/5cax
ftp://data.pdbj.org/pub/pdb/validation_reports/ca/5cax
 Links
Links Assembly
Assembly
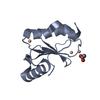



 Components
Components Methanosarcina acetivorans (strain ATCC 35395 / DSM 2834 / JCM 12185 / C2A) (archaea)
Methanosarcina acetivorans (strain ATCC 35395 / DSM 2834 / JCM 12185 / C2A) (archaea)
 X-RAY DIFFRACTION
X-RAY DIFFRACTION Sample preparation
Sample preparation ROTATING ANODE / Type: RIGAKU MICROMAX-007 HF / Wavelength: 1.5418 Å
ROTATING ANODE / Type: RIGAKU MICROMAX-007 HF / Wavelength: 1.5418 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller










 PDBj
PDBj







