+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6wec | ||||||
|---|---|---|---|---|---|---|---|
| Title | Multi-Hit SFX using MHz XFEL sources | ||||||
 Components Components | Lysozyme | ||||||
 Keywords Keywords | HYDROLASE / Enzyme | ||||||
| Function / homology |  Function and homology information Function and homology informationLactose synthesis / Antimicrobial peptides / Neutrophil degranulation / beta-N-acetylglucosaminidase activity / cell wall macromolecule catabolic process / lysozyme / lysozyme activity / defense response to Gram-negative bacterium / killing of cells of another organism / defense response to Gram-positive bacterium ...Lactose synthesis / Antimicrobial peptides / Neutrophil degranulation / beta-N-acetylglucosaminidase activity / cell wall macromolecule catabolic process / lysozyme / lysozyme activity / defense response to Gram-negative bacterium / killing of cells of another organism / defense response to Gram-positive bacterium / defense response to bacterium / endoplasmic reticulum / extracellular space / identical protein binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  FREE ELECTRON LASER / FREE ELECTRON LASER /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.1 Å molecular replacement / Resolution: 2.1 Å | ||||||
 Authors Authors | Holmes, S. / Darmanin, C. / Abbey, B. | ||||||
| Funding support |  Australia, 1items Australia, 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Megahertz pulse trains enable multi-hit serial femtosecond crystallography experiments at X-ray free electron lasers. Authors: Holmes, S. / Kirkwood, H.J. / Bean, R. / Giewekemeyer, K. / Martin, A.V. / Hadian-Jazi, M. / Wiedorn, M.O. / Oberthur, D. / Marman, H. / Adriano, L. / Al-Qudami, N. / Bajt, S. / Barak, I. / ...Authors: Holmes, S. / Kirkwood, H.J. / Bean, R. / Giewekemeyer, K. / Martin, A.V. / Hadian-Jazi, M. / Wiedorn, M.O. / Oberthur, D. / Marman, H. / Adriano, L. / Al-Qudami, N. / Bajt, S. / Barak, I. / Bari, S. / Bielecki, J. / Brockhauser, S. / Coleman, M.A. / Cruz-Mazo, F. / Danilevski, C. / Dorner, K. / Ganan-Calvo, A.M. / Graceffa, R. / Fanghor, H. / Heymann, M. / Frank, M. / Kaukher, A. / Kim, Y. / Kobe, B. / Knoska, J. / Laurus, T. / Letrun, R. / Maia, L. / Messerschmidt, M. / Metz, M. / Michelat, T. / Mills, G. / Molodtsov, S. / Monteiro, D.C.F. / Morgan, A.J. / Munnich, A. / Pena Murillo, G.E. / Previtali, G. / Round, A. / Sato, T. / Schubert, R. / Schulz, J. / Shelby, M. / Seuring, C. / Sellberg, J.A. / Sikorski, M. / Silenzi, A. / Stern, S. / Sztuk-Dambietz, J. / Szuba, J. / Trebbin, M. / Vagovic, P. / Ve, T. / Weinhausen, B. / Wrona, K. / Xavier, P.L. / Xu, C. / Yefanov, O. / Nugent, K.A. / Chapman, H.N. / Mancuso, A.P. / Barty, A. / Abbey, B. / Darmanin, C. #1:  Journal: Nat Commun / Year: 2018 Journal: Nat Commun / Year: 2018Title: Megahertz serial crystallography. Authors: Wiedorn, M.O. / Oberthur, D. / Bean, R. / Schubert, R. / Werner, N. / Abbey, B. / Aepfelbacher, M. / Adriano, L. / Allahgholi, A. / Al-Qudami, N. / Andreasson, J. / Aplin, S. / Awel, S. / ...Authors: Wiedorn, M.O. / Oberthur, D. / Bean, R. / Schubert, R. / Werner, N. / Abbey, B. / Aepfelbacher, M. / Adriano, L. / Allahgholi, A. / Al-Qudami, N. / Andreasson, J. / Aplin, S. / Awel, S. / Ayyer, K. / Bajt, S. / Barak, I. / Bari, S. / Bielecki, J. / Botha, S. / Boukhelef, D. / Brehm, W. / Brockhauser, S. / Cheviakov, I. / Coleman, M.A. / Cruz-Mazo, F. / Danilevski, C. / Darmanin, C. / Doak, R.B. / Domaracky, M. / Dorner, K. / Du, Y. / Fangohr, H. / Fleckenstein, H. / Frank, M. / Fromme, P. / Ganan-Calvo, A.M. / Gevorkov, Y. / Giewekemeyer, K. / Ginn, H.M. / Graafsma, H. / Graceffa, R. / Greiffenberg, D. / Gumprecht, L. / Gottlicher, P. / Hajdu, J. / Hauf, S. / Heymann, M. / Holmes, S. / Horke, D.A. / Hunter, M.S. / Imlau, S. / Kaukher, A. / Kim, Y. / Klyuev, A. / Knoska, J. / Kobe, B. / Kuhn, M. / Kupitz, C. / Kupper, J. / Lahey-Rudolph, J.M. / Laurus, T. / Le Cong, K. / Letrun, R. / Xavier, P.L. / Maia, L. / Maia, F.R.N.C. / Mariani, V. / Messerschmidt, M. / Metz, M. / Mezza, D. / Michelat, T. / Mills, G. / Monteiro, D.C.F. / Morgan, A. / Muhlig, K. / Munke, A. / Munnich, A. / Nette, J. / Nugent, K.A. / Nuguid, T. / Orville, A.M. / Pandey, S. / Pena, G. / Villanueva-Perez, P. / Poehlsen, J. / Previtali, G. / Redecke, L. / Riekehr, W.M. / Rohde, H. / Round, A. / Safenreiter, T. / Sarrou, I. / Sato, T. / Schmidt, M. / Schmitt, B. / Schonherr, R. / Schulz, J. / Sellberg, J.A. / Seibert, M.M. / Seuring, C. / Shelby, M.L. / Shoeman, R.L. / Sikorski, M. / Silenzi, A. / Stan, C.A. / Shi, X. / Stern, S. / Sztuk-Dambietz, J. / Szuba, J. / Tolstikova, A. / Trebbin, M. / Trunk, U. / Vagovic, P. / Ve, T. / Weinhausen, B. / White, T.A. / Wrona, K. / Xu, C. / Yefanov, O. / Zatsepin, N. / Zhang, J. / Perbandt, M. / Mancuso, A.P. / Betzel, C. / Chapman, H. / Barty, A. #2: Journal: Struct Dyn. / Year: 2019 Title: Evaluation of serial crystallographic structure determination within megahertz pulse trains. Authors: Yefanov, O. / Oberthur, D. / Bean, R. / Wiedorn, M.O. / Knoska, J. / Pena, G. / Awel, S. / Gumprecht, L. / Domaracky, M. / Sarrou, I. / Lourdu Xavier, P. / Metz, M. / Bajt, S. / Mariani, V. ...Authors: Yefanov, O. / Oberthur, D. / Bean, R. / Wiedorn, M.O. / Knoska, J. / Pena, G. / Awel, S. / Gumprecht, L. / Domaracky, M. / Sarrou, I. / Lourdu Xavier, P. / Metz, M. / Bajt, S. / Mariani, V. / Gevorkov, Y. / White, T.A. / Tolstikova, A. / Villanueva-Perez, P. / Seuring, C. / Aplin, S. / Estillore, A.D. / Kupper, J. / Klyuev, A. / Kuhn, M. / Laurus, T. / Graafsma, H. / Monteiro, D.C.F. / Trebbin, M. / Maia, F.R.N.C. / Cruz-Mazo, F. / Ganan-Calvo, A.M. / Heymann, M. / Darmanin, C. / Abbey, B. / Schmidt, M. / Fromme, P. / Giewekemeyer, K. / Sikorski, M. / Graceffa, R. / Vagovic, P. / Kluyver, T. / Bergemann, M. / Fangohr, H. / Sztuk-Dambietz, J. / Hauf, S. / Raab, N. / Bondar, V. / Mancuso, A.P. / Chapman, H. / Barty, A. | ||||||
| History |
|
- Structure visualization
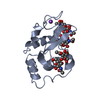
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6wec.cif.gz 6wec.cif.gz | 74.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6wec.ent.gz pdb6wec.ent.gz | 53.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6wec.json.gz 6wec.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6wec_validation.pdf.gz 6wec_validation.pdf.gz | 445.3 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6wec_full_validation.pdf.gz 6wec_full_validation.pdf.gz | 445.5 KB | Display | |
| Data in XML |  6wec_validation.xml.gz 6wec_validation.xml.gz | 7.8 KB | Display | |
| Data in CIF |  6wec_validation.cif.gz 6wec_validation.cif.gz | 9.7 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/we/6wec https://data.pdbj.org/pub/pdb/validation_reports/we/6wec ftp://data.pdbj.org/pub/pdb/validation_reports/we/6wec ftp://data.pdbj.org/pub/pdb/validation_reports/we/6wec | HTTPS FTP |
-Related structure data
| Related structure data |  6webC  7tumC  6ftrS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 14331.160 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| #2: Chemical | | #3: Chemical | ChemComp-EDO / #4: Chemical | ChemComp-ACT / #5: Water | ChemComp-HOH / | Has ligand of interest | N | Has protein modification | Y | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.07 Å3/Da / Density % sol: 40.56 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: batch mode / pH: 3.5 Details: NaCl, ethylene glycol, PEG 3350, acetate buffer pH 3.5 |
-Data collection
| Diffraction | Mean temperature: 293 K / Serial crystal experiment: Y |
|---|---|
| Diffraction source | Source:  FREE ELECTRON LASER / Site: FREE ELECTRON LASER / Site:  European XFEL European XFEL  / Beamline: SPB/SFX / Wavelength: 1.3332 Å / Beamline: SPB/SFX / Wavelength: 1.3332 Å |
| Detector | Type: AGIPD / Detector: PIXEL / Date: Sep 14, 2017 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.3332 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→21.66 Å / Num. obs: 7310 / % possible obs: 98.5 % / Redundancy: 1 % / CC1/2: 0.615 / Net I/σ(I): 2.9 |
| Reflection shell | Resolution: 2.1→2.155 Å / Num. unique obs: 494 / CC1/2: 0.372 / % possible all: 92.34 |
| Serial crystallography sample delivery | Method: injection |
-Phasing
| Phasing | Method:  molecular replacement molecular replacement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phasing MR |
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6FTR Resolution: 2.1→21.66 Å / Cor.coef. Fo:Fc: 0.885 / Cor.coef. Fo:Fc free: 0.834 / SU B: 20.89 / SU ML: 0.258 / Cross valid method: THROUGHOUT / ESU R Free: 0.275 Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 56.55 Å2 / Biso mean: 20.395 Å2 / Biso min: 11.11 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.1→21.66 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj











