| Entry | Database: PDB / ID: 6vtr
|
|---|
| Title | Crystal structure of G16S human Galectin-7 mutant |
|---|
 Components Components | Galectin-7 |
|---|
 Keywords Keywords | SUGAR BINDING PROTEIN / Human Galectin-7 / lactose-furanose |
|---|
| Function / homology |  Function and homology information Function and homology information
Differentiation of Keratinocytes in Interfollicular Epidermis in Mammalian Skin / heterophilic cell-cell adhesion / carbohydrate binding / apoptotic process / extracellular space / extracellular exosome / nucleus / cytoplasmSimilarity search - Function Galectin-like / Galactoside-binding lectin / Galectin / Galectin, carbohydrate recognition domain / Galactoside-binding lectin / Galactoside-binding lectin (galectin) domain profile. / Jelly Rolls - #200 / Concanavalin A-like lectin/glucanase domain superfamily / Jelly Rolls / Sandwich / Mainly BetaSimilarity search - Domain/homology |
|---|
| Biological species |  Homo sapiens (human) Homo sapiens (human) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.3 Å molecular replacement / Resolution: 2.3 Å |
|---|
 Authors Authors | Pham, N.T.H. / Calmettes, C. / Doucet, N. |
|---|
| Funding support |  Canada, Canada,  United States, 6items United States, 6items | Organization | Grant number | Country |
|---|
| Natural Sciences and Engineering Research Council (NSERC, Canada) | RGPIN 2016-05557 |  Canada Canada | | National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | R01GM105978 |  United States United States | | Fonds de Recherche du Quebec - Sante (FRQS) | Research Scholar Senior Career Award (281993) |  Canada Canada | | Fonds de Recherche du Quebec - Sante (FRQS) | Junior 1 (251848) |  Canada Canada | | RGPIN-2017-06091 |  Canada Canada | | Fonds de Recherche du Quebec - Sante (FRQS) | Doctoral Training scholarship (287239) |  Canada Canada |
|
|---|
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2021 Journal: J.Biol.Chem. / Year: 2021
Title: Perturbing dimer interactions and allosteric communication modulates the immunosuppressive activity of human galectin-7.
Authors: Pham, N.T.H. / Letourneau, M. / Fortier, M. / Begin, G. / Al-Abdul-Wahid, M.S. / Pucci, F. / Folch, B. / Rooman, M. / Chatenet, D. / St-Pierre, Y. / Lague, P. / Calmettes, C. / Doucet, N. |
|---|
| History | | Deposition | Feb 13, 2020 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | Aug 25, 2021 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Nov 17, 2021 | Group: Database references / Category: citation / citation_author
Item: _citation.country / _citation.journal_abbrev ..._citation.country / _citation.journal_abbrev / _citation.journal_id_ASTM / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year |
|---|
| Revision 1.2 | Nov 24, 2021 | Group: Database references / Category: citation / Item: _citation.journal_volume |
|---|
| Revision 1.3 | Oct 11, 2023 | Group: Data collection / Refinement description
Category: chem_comp_atom / chem_comp_bond / pdbx_initial_refinement_model |
|---|
|
|---|
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT /
MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.3 Å
molecular replacement / Resolution: 2.3 Å  Authors
Authors Canada,
Canada,  United States, 6items
United States, 6items  Citation
Citation Journal: J.Biol.Chem. / Year: 2021
Journal: J.Biol.Chem. / Year: 2021 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 6vtr.cif.gz
6vtr.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb6vtr.ent.gz
pdb6vtr.ent.gz PDB format
PDB format 6vtr.json.gz
6vtr.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/vt/6vtr
https://data.pdbj.org/pub/pdb/validation_reports/vt/6vtr ftp://data.pdbj.org/pub/pdb/validation_reports/vt/6vtr
ftp://data.pdbj.org/pub/pdb/validation_reports/vt/6vtr
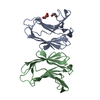



 Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: LGALS7, PIG1, LGALS7B / Production host:
Homo sapiens (human) / Gene: LGALS7, PIG1, LGALS7B / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  CLSI
CLSI  / Beamline: 08B1-1 / Wavelength: 1.0332 Å
/ Beamline: 08B1-1 / Wavelength: 1.0332 Å molecular replacement
molecular replacement Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller



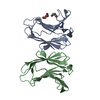









 PDBj
PDBj



