+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6uhj | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | X-ray Structure of C148 mGFP | |||||||||
 Components Components | C148 mGFP | |||||||||
 Keywords Keywords | FLUORESCENT PROTEIN / green fluorescent protein / beta-barrel | |||||||||
| Function / homology | Green fluorescent protein, GFP / Green fluorescent protein-related / Green fluorescent protein / Green fluorescent protein / bioluminescence / generation of precursor metabolites and energy / Green fluorescent protein Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.5 Å MOLECULAR REPLACEMENT / Resolution: 1.5 Å | |||||||||
 Authors Authors | Winegar, P.W. / Hayes, O.G. / McMillan, J.R. / Figg, C.A. / Focia, P.J. / Mirkin, C.A. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Chem / Year: 2020 Journal: Chem / Year: 2020Title: DNA-Directed Protein Packing within Single Crystals. Authors: Winegar, P.H. / Hayes, O.G. / McMillan, J.R. / Figg, C.A. / Focia, P.J. / Mirkin, C.A. | |||||||||
| History |
|
- Structure visualization
Structure visualization
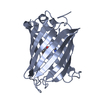
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6uhj.cif.gz 6uhj.cif.gz | 71.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6uhj.ent.gz pdb6uhj.ent.gz | 48.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6uhj.json.gz 6uhj.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/uh/6uhj https://data.pdbj.org/pub/pdb/validation_reports/uh/6uhj ftp://data.pdbj.org/pub/pdb/validation_reports/uh/6uhj ftp://data.pdbj.org/pub/pdb/validation_reports/uh/6uhj | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6uhkC  6uhlC  6uhmC  6uhnC  6uhoC  6uhpC  6uhqC  6uhrC  5n9oS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 31038.908 Da / Num. of mol.: 1 / Mutation: S148C Source method: isolated from a genetically manipulated source Details: This mutant of EGFP has a single surface cysteine at residue 148 (counting from M37 and counting CRO as 3 residues) and an N-terminal his-tag. Source: (gene. exp.)   |
|---|---|
| #2: Water | ChemComp-HOH / |
| Has ligand of interest | N |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.81 Å3/Da / Density % sol: 32.08 % / Description: Green crystal with sharp facets |
|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, sitting drop / pH: 7.4 Details: 1 microliter C148 mGFP (5 mg/mL of protein in 10 mM Tris Buffer pH 7.4, 137 mM NaCl) + 1 microliter crystallization condition (0.1 M sodium acetate, 25% (w/v) PEG 4000, 8% (v/v) isopropanol) ...Details: 1 microliter C148 mGFP (5 mg/mL of protein in 10 mM Tris Buffer pH 7.4, 137 mM NaCl) + 1 microliter crystallization condition (0.1 M sodium acetate, 25% (w/v) PEG 4000, 8% (v/v) isopropanol) in a sitting drop with a 70 microliter reservoir (0.1 M sodium acetate, 25% (w/v) PEG 4000, 8% (v/v) isopropanol) |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 21-ID-G / Wavelength: 0.97857 Å / Beamline: 21-ID-G / Wavelength: 0.97857 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: MAR scanner 300 mm plate / Detector: IMAGE PLATE / Date: Oct 3, 2018 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: C(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.97857 Å / Relative weight: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.5→51.51 Å / Num. obs: 36338 / % possible obs: 98.8 % / Redundancy: 4.5 % / Rpim(I) all: 0.043 / Rrim(I) all: 0.094 / Rsym value: 0.083 / Net I/av σ(I): 6 / Net I/σ(I): 10.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5N9O Resolution: 1.5→46.61 Å / Cor.coef. Fo:Fc: 0.965 / Cor.coef. Fo:Fc free: 0.953 / SU B: 1.139 / SU ML: 0.043 / SU R Cruickshank DPI: 0.0687 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.069 / ESU R Free: 0.072 Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 57.23 Å2 / Biso mean: 10.97 Å2 / Biso min: 4.87 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.5→46.61 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.501→1.54 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller






















 PDBj
PDBj


