[English] 日本語
 Yorodumi
Yorodumi- PDB-6r37: T. brucei FPPS in complex with 2-(5-chlorobenzo[b]thiophen-3-yl)a... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6r37 | ||||||
|---|---|---|---|---|---|---|---|
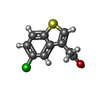
| Title | T. brucei FPPS in complex with 2-(5-chlorobenzo[b]thiophen-3-yl)acetic acid | ||||||
 Components Components | Farnesyl pyrophosphate synthase | ||||||
 Keywords Keywords | TRANSFERASE / sterol biosynthesis / farnesyl diphosphate synthase / Trypanosoma brucei / homodimer | ||||||
| Function / homology |  Function and homology information Function and homology informationfarnesyl diphosphate biosynthetic process / dimethylallyltranstransferase activity / (2E,6E)-farnesyl diphosphate synthase activity / metal ion binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | ||||||
 Authors Authors | Muenzker, L. / Petrick, J.K. / Schleberger, C. / Jahnke, W. | ||||||
| Funding support |  Switzerland, 1items Switzerland, 1items
| ||||||
 Citation Citation |  Journal: Thesis / Year: 2019 Journal: Thesis / Year: 2019Title: Targeting Trypanosoma brucei FPPS by Fragment-based drug discovery Authors: Muenzker, L. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6r37.cif.gz 6r37.cif.gz | 149.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6r37.ent.gz pdb6r37.ent.gz | 117.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6r37.json.gz 6r37.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/r3/6r37 https://data.pdbj.org/pub/pdb/validation_reports/r3/6r37 ftp://data.pdbj.org/pub/pdb/validation_reports/r3/6r37 ftp://data.pdbj.org/pub/pdb/validation_reports/r3/6r37 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6r38C  4rypS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 42169.211 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: GP is an expression-tag / Source: (gene. exp.)   |
|---|---|
| #2: Chemical | ChemComp-3N2 / ( |
| #3: Chemical | ChemComp-DMS / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.16 Å3/Da / Density % sol: 43.17 % / Description: thin needles |
|---|---|
| Crystal grow | Temperature: 293.15 K / Method: vapor diffusion, sitting drop Details: 0.12 M Cesium chloride, 12 %w/v PEG 3350, 12 % v/v DMSO |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X10SA / Wavelength: 0.99996 Å / Beamline: X10SA / Wavelength: 0.99996 Å |
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: Nov 25, 2017 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.99996 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→52.628 Å / Num. obs: 21086 / % possible obs: 91.3 % / Redundancy: 18.2 % / Biso Wilson estimate: 56.15 Å2 / CC1/2: 1 / Rmerge(I) obs: 0.08 / Rpim(I) all: 0.02 / Rrim(I) all: 0.083 / Rsym value: 0.08 / Net I/σ(I): 19.4 |
| Reflection shell | Resolution: 2.1→2.136 Å / Redundancy: 17.4 % / Rmerge(I) obs: 4.686 / Mean I/σ(I) obs: 0.6 / Num. unique obs: 1075 / CC1/2: 0.466 / Rpim(I) all: 1.135 / Rrim(I) all: 4.827 / Rsym value: 4.686 / % possible all: 98.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4ryp Resolution: 2.1→52.628 Å / Cor.coef. Fo:Fc: 0.942 / Cor.coef. Fo:Fc free: 0.928 / SU R Cruickshank DPI: 0.257 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.268 / SU Rfree Blow DPI: 0.198 / SU Rfree Cruickshank DPI: 0.195
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 219.29 Å2 / Biso mean: 92.83 Å2 / Biso min: 44.87 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.41 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.1→52.628 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.1→2.11 Å / Rfactor Rfree error: 0 / Total num. of bins used: 50
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 3.2875 Å / Origin y: 11.8605 Å / Origin z: -11.2889 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: { A|* } |
 Movie
Movie Controller
Controller












 PDBj
PDBj