Entry Database : PDB / ID : 6q9nTitle Crystal structure of PBP2a from MRSA in complex with piperacillin and quinazolinone Penicillin binding protein 2 prime Keywords / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Staphylococcus aureus (bacteria)Method / / / Resolution : 2.5 Å Authors Martinez-Caballero, S. / Batuecas, M.T. / Hermoso, J.A. Funding support Organization Grant number Country National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID) 1R01AI116548
Journal : Antimicrob.Agents Chemother. / Year : 2019Title : The Quinazolinone Allosteric Inhibitor of PBP 2a Synergizes with Piperacillin and Tazobactam against Methicillin-Resistant Staphylococcus aureus.Authors : Janardhanan, J. / Bouley, R. / Martinez-Caballero, S. / Peng, Z. / Batuecas-Mordillo, M. / Meisel, J.E. / Ding, D. / Schroeder, V.A. / Wolter, W.R. / Mahasenan, K.V. / Hermoso, J.A. / Mobashery, S. / Chang, M. History Deposition Dec 18, 2018 Deposition site / Processing site Revision 1.0 Nov 27, 2019 Provider / Type Revision 1.1 Jul 29, 2020 Group / Derived calculations / Structure summaryCategory chem_comp / entity ... chem_comp / entity / pdbx_chem_comp_identifier / pdbx_entity_nonpoly / struct_conn / struct_conn_type / struct_site / struct_site_gen Item _chem_comp.name / _chem_comp.type ... _chem_comp.name / _chem_comp.type / _entity.pdbx_description / _pdbx_entity_nonpoly.name / _struct_conn.conn_type_id / _struct_conn.id / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id / _struct_conn_type.id Description / Provider / Type Revision 1.2 Sep 9, 2020 Group / Category / Item Revision 1.3 Mar 30, 2022 Group / Database references / Category / pdbx_audit_supportItem / _database_2.pdbx_database_accession / _pdbx_audit_support.funding_organizationRevision 1.4 Jan 24, 2024 Group / Refinement descriptionCategory / chem_comp_bond / pdbx_initial_refinement_modelRevision 1.5 Oct 16, 2024 Group / Category / pdbx_modification_feature
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.5 Å
MOLECULAR REPLACEMENT / Resolution: 2.5 Å  Authors
Authors United States, 1items
United States, 1items  Citation
Citation Journal: Antimicrob.Agents Chemother. / Year: 2019
Journal: Antimicrob.Agents Chemother. / Year: 2019 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 6q9n.cif.gz
6q9n.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb6q9n.ent.gz
pdb6q9n.ent.gz PDB format
PDB format 6q9n.json.gz
6q9n.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/q9/6q9n
https://data.pdbj.org/pub/pdb/validation_reports/q9/6q9n ftp://data.pdbj.org/pub/pdb/validation_reports/q9/6q9n
ftp://data.pdbj.org/pub/pdb/validation_reports/q9/6q9n

 Links
Links Assembly
Assembly


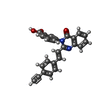
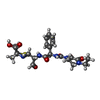
 Components
Components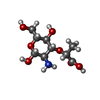

 Staphylococcus aureus (strain Mu50 / ATCC 700699) (bacteria)
Staphylococcus aureus (strain Mu50 / ATCC 700699) (bacteria)







 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  ALBA
ALBA  / Beamline: XALOC / Wavelength: 0.979257 Å
/ Beamline: XALOC / Wavelength: 0.979257 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller












 PDBj
PDBj




