[English] 日本語
 Yorodumi
Yorodumi- PDB-6ps5: XFEL beta2 AR structure by ligand exchange from Timolol to Propra... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6ps5 | ||||||
|---|---|---|---|---|---|---|---|
| Title | XFEL beta2 AR structure by ligand exchange from Timolol to Propranolol. | ||||||
 Components Components | Fusion protein of Beta-2 adrenergic receptor and T4 Lysozyme | ||||||
 Keywords Keywords | MEMBRANE PROTEIN / GPCR / COMPLEX-LCP method / SBDD / drug design / XFEL / LCP-SFX / Ligand Exchange / Timolol / Propranolol / b2AR / beta2AR | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of mini excitatory postsynaptic potential / beta2-adrenergic receptor activity / negative regulation of smooth muscle contraction / AMPA selective glutamate receptor signaling pathway / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of autophagosome maturation / heat generation / norepinephrine binding / Adrenoceptors / positive regulation of lipophagy ...positive regulation of mini excitatory postsynaptic potential / beta2-adrenergic receptor activity / negative regulation of smooth muscle contraction / AMPA selective glutamate receptor signaling pathway / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of autophagosome maturation / heat generation / norepinephrine binding / Adrenoceptors / positive regulation of lipophagy / negative regulation of G protein-coupled receptor signaling pathway / negative regulation of multicellular organism growth / response to psychosocial stress / adrenergic receptor signaling pathway / endosome to lysosome transport / diet induced thermogenesis / positive regulation of cAMP/PKA signal transduction / adenylate cyclase binding / smooth muscle contraction / potassium channel regulator activity / bone resorption / positive regulation of bone mineralization / neuronal dense core vesicle / viral release from host cell by cytolysis / intercellular bridge / regulation of sodium ion transport / adenylate cyclase-activating adrenergic receptor signaling pathway / peptidoglycan catabolic process / brown fat cell differentiation / receptor-mediated endocytosis / response to cold / clathrin-coated endocytic vesicle membrane / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / cellular response to amyloid-beta / cell wall macromolecule catabolic process / lysozyme / lysozyme activity / mitotic spindle / Cargo recognition for clathrin-mediated endocytosis / positive regulation of cold-induced thermogenesis / amyloid-beta binding / Clathrin-mediated endocytosis / microtubule cytoskeleton / G alpha (s) signalling events / transcription by RNA polymerase II / host cell cytoplasm / early endosome / cell surface receptor signaling pathway / lysosome / receptor complex / positive regulation of MAPK cascade / apical plasma membrane / endosome / endosome membrane / defense response to bacterium / Ub-specific processing proteases / cilium / ciliary basal body / protein-containing complex binding / Golgi apparatus / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / identical protein binding / membrane / nucleus / plasma membrane Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) Enterobacteria phage T4 (virus) Enterobacteria phage T4 (virus) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  FREE ELECTRON LASER / FREE ELECTRON LASER /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.9 Å molecular replacement / Resolution: 2.9 Å | ||||||
 Authors Authors | Ishchenko, A. / Stauch, B. / Han, G.W. / Batyuk, A. / Shiriaeva, A. / Li, C. / Zatsepin, N.A. / Weierstall, U. / Liu, W. / Nango, E. ...Ishchenko, A. / Stauch, B. / Han, G.W. / Batyuk, A. / Shiriaeva, A. / Li, C. / Zatsepin, N.A. / Weierstall, U. / Liu, W. / Nango, E. / Nakane, T. / Tanaka, R. / Tono, K. / Joti, Y. / Iwata, S. / Moraes, I. / Gati, C. / Cherezov, C. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Iucrj / Year: 2019 Journal: Iucrj / Year: 2019Title: Toward G protein-coupled receptor structure-based drug design using X-ray lasers. Authors: Ishchenko, A. / Stauch, B. / Han, G.W. / Batyuk, A. / Shiriaeva, A. / Li, C. / Zatsepin, N. / Weierstall, U. / Liu, W. / Nango, E. / Nakane, T. / Tanaka, R. / Tono, K. / Joti, Y. / Iwata, S. ...Authors: Ishchenko, A. / Stauch, B. / Han, G.W. / Batyuk, A. / Shiriaeva, A. / Li, C. / Zatsepin, N. / Weierstall, U. / Liu, W. / Nango, E. / Nakane, T. / Tanaka, R. / Tono, K. / Joti, Y. / Iwata, S. / Moraes, I. / Gati, C. / Cherezov, V. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6ps5.cif.gz 6ps5.cif.gz | 197.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6ps5.ent.gz pdb6ps5.ent.gz | 153.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6ps5.json.gz 6ps5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ps/6ps5 https://data.pdbj.org/pub/pdb/validation_reports/ps/6ps5 ftp://data.pdbj.org/pub/pdb/validation_reports/ps/6ps5 ftp://data.pdbj.org/pub/pdb/validation_reports/ps/6ps5 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6przC  6ps0C  6ps1C  6ps2C  6ps3C  6ps4C  6ps6C  6ps7C  6ps8C  3d4sS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Details | AUTHORS STATE THAT THE BIOLOGICAL UNIT IS UNKNOWN |
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 57774.246 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human), (gene. exp.) Homo sapiens (human), (gene. exp.)  Enterobacteria phage T4 (virus) Enterobacteria phage T4 (virus)Gene: ADRB2, ADRB2R, B2AR, e, T4Tp126 / Production host:  |
|---|
-Non-polymers , 5 types, 12 molecules 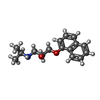








| #2: Chemical | ChemComp-SNP / | ||||||
|---|---|---|---|---|---|---|---|
| #3: Chemical | ChemComp-SO4 / #4: Chemical | ChemComp-CLR / | #5: Chemical | #6: Chemical | ChemComp-OLC / ( |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.49 Å3/Da / Density % sol: 50.7 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: lipidic cubic phase Details: 0.1 M HEPES pH 7.0, 0.1 M Ammonium Sulfate, 30% PEG 400, 2 mM of target ligand Propranolol |
-Data collection
| Diffraction | Mean temperature: 293 K / Serial crystal experiment: Y |
|---|---|
| Diffraction source | Source:  FREE ELECTRON LASER / Site: FREE ELECTRON LASER / Site:  SACLA SACLA  / Beamline: BL3 / Wavelength: 1.76 Å / Beamline: BL3 / Wavelength: 1.76 Å |
| Detector | Type: MPCCD / Detector: CCD / Date: May 19, 2015 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.76 Å / Relative weight: 1 |
| Reflection | Resolution: 2.9→46.44 Å / Num. obs: 13464 / % possible obs: 100 % / Redundancy: 104 % / CC1/2: 0.961 / R split: 0.233 / Net I/σ(I): 2.8 |
| Reflection shell | Resolution: 2.9→3.13 Å / Num. unique obs: 2674 / CC1/2: 0.225 / R split: 1.854 |
| Serial crystallography measurement | Collection time total: 1.08 hours / Focal spot size: 1.5 µm2 / Pulse duration: 10 fsec. / Pulse photon energy: 7 keV / XFEL pulse repetition rate: 30 Hz |
| Serial crystallography sample delivery | Method: injection |
| Serial crystallography data reduction | Crystal hits: 8494 / Frames indexed: 5201 / Frames total: 117972 |
-Phasing
| Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3d4s Resolution: 2.9→46.44 Å / Cor.coef. Fo:Fc: 0.932 / Cor.coef. Fo:Fc free: 0.91 / Rfactor Rfree error: 0 / Cross valid method: THROUGHOUT / σ(F): 0 / SU Rfree Blow DPI: 0.384
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 186.23 Å2 / Biso mean: 105.25 Å2 / Biso min: 76.13 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.52 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.9→46.44 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.9→3.13 Å / Rfactor Rfree error: 0 / Total num. of bins used: 7
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 8.4218 Å / Origin y: 3.0831 Å / Origin z: 26.0663 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: { A|* } |
 Movie
Movie Controller
Controller












 PDBj
PDBj


















