+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6njm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Architecture and subunit arrangement of native AMPA receptors | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN/Immune System / AMPA receptor / ligand gated ion channel / neurotransmitter / synapse / MEMBRANE PROTEIN / MEMBRANE PROTEIN-Immune System complex | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationPresynaptic depolarization and calcium channel opening / eye blink reflex / positive regulation of protein localization to basolateral plasma membrane / cerebellar mossy fiber / postsynaptic neurotransmitter receptor diffusion trapping / channel regulator activity / LGI-ADAM interactions / Trafficking of AMPA receptors / regulation of AMPA receptor activity / membrane hyperpolarization ...Presynaptic depolarization and calcium channel opening / eye blink reflex / positive regulation of protein localization to basolateral plasma membrane / cerebellar mossy fiber / postsynaptic neurotransmitter receptor diffusion trapping / channel regulator activity / LGI-ADAM interactions / Trafficking of AMPA receptors / regulation of AMPA receptor activity / membrane hyperpolarization / nervous system process / Synaptic adhesion-like molecules / protein targeting to membrane / voltage-gated calcium channel complex / spine synapse / dendritic spine neck / dendritic spine cytoplasm / cellular response to amine stimulus / dendritic spine head / protein heterotetramerization / neurotransmitter receptor localization to postsynaptic specialization membrane / perisynaptic space / Activation of AMPA receptors / ligand-gated monoatomic cation channel activity / neuromuscular junction development / AMPA glutamate receptor activity / Trafficking of GluR2-containing AMPA receptors / parallel fiber to Purkinje cell synapse / response to lithium ion / transmission of nerve impulse / AMPA glutamate receptor clustering / cellular response to glycine / kainate selective glutamate receptor activity / AMPA glutamate receptor complex / immunoglobulin binding / asymmetric synapse / regulation of receptor recycling / extracellularly glutamate-gated ion channel activity / ionotropic glutamate receptor complex / membrane depolarization / conditioned place preference / Unblocking of NMDA receptors, glutamate binding and activation / glutamate receptor binding / positive regulation of synaptic transmission / regulation of synaptic transmission, glutamatergic / positive regulation of synaptic transmission, glutamatergic / regulation of postsynaptic membrane neurotransmitter receptor levels / response to fungicide / voltage-gated calcium channel activity / synaptic cleft / cytoskeletal protein binding / extracellular ligand-gated monoatomic ion channel activity / glutamate-gated receptor activity / cellular response to brain-derived neurotrophic factor stimulus / regulation of long-term synaptic depression / somatodendritic compartment / glutamate-gated calcium ion channel activity / presynaptic active zone membrane / dendrite membrane / excitatory synapse / ionotropic glutamate receptor binding / ionotropic glutamate receptor signaling pathway / dendrite cytoplasm / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / synaptic membrane / positive regulation of excitatory postsynaptic potential / dendritic shaft / hippocampal mossy fiber to CA3 synapse / SNARE binding / PDZ domain binding / calcium channel regulator activity / synaptic transmission, glutamatergic / regulation of membrane potential / protein tetramerization / establishment of protein localization / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / response to calcium ion / cerebral cortex development / postsynaptic density membrane / receptor internalization / modulation of chemical synaptic transmission / Schaffer collateral - CA1 synapse / terminal bouton / synaptic vesicle / long-term synaptic potentiation / synaptic vesicle membrane / signaling receptor activity / amyloid-beta binding / presynapse / growth cone / presynaptic membrane / scaffold protein binding / dendritic spine / chemical synaptic transmission / protein homotetramerization / perikaryon / postsynaptic membrane / neuron projection / postsynaptic density / axon Similarity search - Function | ||||||||||||
| Biological species |   | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 6.5 Å | ||||||||||||
 Authors Authors | Gouaux, E. / Zhao, Y. | ||||||||||||
 Citation Citation |  Journal: Science / Year: 2019 Journal: Science / Year: 2019Title: Architecture and subunit arrangement of native AMPA receptors elucidated by cryo-EM. Authors: Yan Zhao / Shanshuang Chen / Adam C Swensen / Wei-Jun Qian / Eric Gouaux /  Abstract: Glutamate-gated AMPA receptors mediate the fast component of excitatory signal transduction at chemical synapses throughout all regions of the mammalian brain. AMPA receptors are tetrameric ...Glutamate-gated AMPA receptors mediate the fast component of excitatory signal transduction at chemical synapses throughout all regions of the mammalian brain. AMPA receptors are tetrameric assemblies composed of four subunits, GluA1-GluA4. Despite decades of study, the subunit composition, subunit arrangement, and molecular structure of native AMPA receptors remain unknown. Here we elucidate the structures of 10 distinct native AMPA receptor complexes by single-particle cryo-electron microscopy (cryo-EM). We find that receptor subunits are arranged nonstochastically, with the GluA2 subunit preferentially occupying the B and D positions of the tetramer and with triheteromeric assemblies comprising a major population of native AMPA receptors. Cryo-EM maps define the structure for S2-M4 linkers between the ligand-binding and transmembrane domains, suggesting how neurotransmitter binding is coupled to ion channel gating. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6njm.cif.gz 6njm.cif.gz | 898.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6njm.ent.gz pdb6njm.ent.gz | 693.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6njm.json.gz 6njm.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/nj/6njm https://data.pdbj.org/pub/pdb/validation_reports/nj/6njm ftp://data.pdbj.org/pub/pdb/validation_reports/nj/6njm ftp://data.pdbj.org/pub/pdb/validation_reports/nj/6njm | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  9388MC  0426C  0427C  0428C  0429C  0430C  0431C  0432C  9387C  9389C  6njlC  6njnC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Glutamate receptor ... , 2 types, 4 molecules ACBD
| #1: Protein | Mass: 100556.680 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #2: Protein | Mass: 98783.805 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  |
|---|
-Antibody , 5 types, 10 molecules EGIMJNKOLP
| #3: Antibody | Mass: 13039.064 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #5: Antibody | Mass: 18570.826 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #6: Antibody | Mass: 21038.842 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #7: Antibody | Mass: 25111.660 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #8: Antibody | Mass: 27975.439 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Protein / Non-polymers , 2 types, 6 molecules FH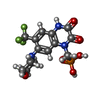

| #11: Chemical | ChemComp-ZK1 / {[ #4: Protein | Mass: 35938.746 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  |
|---|
-Sugars , 3 types, 10 molecules 
| #9: Polysaccharide | beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta- ...beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #10: Polysaccharide | Source method: isolated from a genetically manipulated source #12: Sugar | ChemComp-NAG / |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source (natural) |
| ||||||||||||||||||||||||
| Buffer solution | pH: 8 | ||||||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||||||
| Specimen | Conc.: 4 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||
| Specimen support | Details: unspecified | ||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 295 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 54 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| EM software |
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||
| 3D reconstruction | Resolution: 6.5 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 99000 / Symmetry type: POINT | ||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT / Space: REAL |
 Movie
Movie Controller
Controller










 PDBj
PDBj









