[English] 日本語
 Yorodumi
Yorodumi- PDB-6nbt: CRISPR Complex Subunit Csm3 from Staphylococcus epidermidis RP62a -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6nbt | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRISPR Complex Subunit Csm3 from Staphylococcus epidermidis RP62a | ||||||
 Components Components | CRISPR-associated protein | ||||||
 Keywords Keywords | RNA BINDING PROTEIN / CRISPR / RNA Binding / RNA Recognition Motif / Samarium (III) chloride | ||||||
| Function / homology |  Function and homology information Function and homology informationendonuclease activity / defense response to virus / hydrolase activity / RNA binding / metal ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 2.4 Å SAD / Resolution: 2.4 Å | ||||||
 Authors Authors | Dorsey, B.W. / Mondragon, A. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2019 Journal: Nucleic Acids Res / Year: 2019Title: Structural organization of a Type III-A CRISPR effector subcomplex determined by X-ray crystallography and cryo-EM. Authors: Bryan W Dorsey / Lei Huang / Alfonso Mondragón /  Abstract: Clustered regularly interspaced short palindromic repeats (CRISPR) and their associated Cas proteins provide an immune-like response in many prokaryotes against extraneous nucleic acids. CRISPR-Cas ...Clustered regularly interspaced short palindromic repeats (CRISPR) and their associated Cas proteins provide an immune-like response in many prokaryotes against extraneous nucleic acids. CRISPR-Cas systems are classified into different classes and types. Class 1 CRISPR-Cas systems form multi-protein effector complexes that includes a guide RNA (crRNA) used to identify the target for destruction. Here we present crystal structures of Staphylococcus epidermidis Type III-A CRISPR subunits Csm2 and Csm3 and a 5.2 Å resolution single-particle cryo-electron microscopy (cryo-EM) reconstruction of an in vivo assembled effector subcomplex including the crRNA. The structures help to clarify the quaternary architecture of Type III-A effector complexes, and provide details on crRNA binding, target RNA binding and cleavage, and intermolecular interactions essential for effector complex assembly. The structures allow a better understanding of the organization of Type III-A CRISPR effector complexes as well as highlighting the overall similarities and differences with other Class 1 effector complexes. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6nbt.cif.gz 6nbt.cif.gz | 92.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6nbt.ent.gz pdb6nbt.ent.gz | 68.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6nbt.json.gz 6nbt.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/nb/6nbt https://data.pdbj.org/pub/pdb/validation_reports/nb/6nbt ftp://data.pdbj.org/pub/pdb/validation_reports/nb/6nbt ftp://data.pdbj.org/pub/pdb/validation_reports/nb/6nbt | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 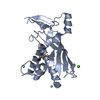
| ||||||||||||||||||
| 2 | 
| ||||||||||||||||||
| 3 | 
| ||||||||||||||||||
| Unit cell |
| ||||||||||||||||||
| Components on special symmetry positions |
| ||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: _ / Ens-ID: 1 / Beg auth comp-ID: TYR / Beg label comp-ID: TYR / End auth comp-ID: LYS / End label comp-ID: LYS / Refine code: _ / Auth seq-ID: 2 - 214 / Label seq-ID: 26 - 238
|
- Components
Components
| #1: Protein | Mass: 27012.383 Da / Num. of mol.: 2 / Fragment: Subunit Csm3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Staphylococcus epidermidis (strain ATCC 35984 / RP62A) (bacteria) Staphylococcus epidermidis (strain ATCC 35984 / RP62A) (bacteria)Strain: ATCC 35984 / RP62A / Cell line: 35984D-5 / Gene: SERP2459 / Plasmid: pMCSG7 / Production host:  #2: Chemical | #3: Chemical | ChemComp-SM / #4: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.03 Å3/Da / Density % sol: 39.51 % |
|---|---|
| Crystal grow | Temperature: 287 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: PEG 8000, calcium acetate, 2-(N-morpholino)ethanesulfonic acid (MES) |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 21-ID-D / Wavelength: 1.0781 Å / Beamline: 21-ID-D / Wavelength: 1.0781 Å |
| Detector | Type: DECTRIS EIGER X 9M / Detector: PIXEL / Date: Feb 28, 2018 |
| Radiation | Monochromator: Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.0781 Å / Relative weight: 1 |
| Reflection | Resolution: 2.4→46.57 Å / Num. obs: 16891 / % possible obs: 98.2 % / Redundancy: 6.8 % / Net I/σ(I): 10.3 |
| Reflection shell | Resolution: 2.4→2.49 Å |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SAD / Resolution: 2.4→25 Å / Cor.coef. Fo:Fc: 0.939 / Cor.coef. Fo:Fc free: 0.921 / SU B: 10.897 / SU ML: 0.246 / Cross valid method: THROUGHOUT / ESU R: 0.491 / ESU R Free: 0.284 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS SAD / Resolution: 2.4→25 Å / Cor.coef. Fo:Fc: 0.939 / Cor.coef. Fo:Fc free: 0.921 / SU B: 10.897 / SU ML: 0.246 / Cross valid method: THROUGHOUT / ESU R: 0.491 / ESU R Free: 0.284 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 50.19 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 2.4→25 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj





