[English] 日本語
 Yorodumi
Yorodumi- PDB-6g79: Coupling specificity of heterotrimeric Go to the serotonin 5-HT1B... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6g79 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Coupling specificity of heterotrimeric Go to the serotonin 5-HT1B receptor | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | MEMBRANE PROTEIN / G-protein coupled receptor / 5-HT1B / Mini-Go / serotonin | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of serotonin secretion / serotonergic synapse / Gi/o-coupled serotonin receptor activity / G protein-coupled serotonin receptor complex / regulation of behavior / phospholipase C-activating serotonin receptor signaling pathway / serotonin receptor activity / Serotonin receptors / G protein-coupled serotonin receptor activity / vasoconstriction ...negative regulation of serotonin secretion / serotonergic synapse / Gi/o-coupled serotonin receptor activity / G protein-coupled serotonin receptor complex / regulation of behavior / phospholipase C-activating serotonin receptor signaling pathway / serotonin receptor activity / Serotonin receptors / G protein-coupled serotonin receptor activity / vasoconstriction / neurotransmitter receptor activity / mu-type opioid receptor binding / bone remodeling / corticotropin-releasing hormone receptor 1 binding / serotonin binding / vesicle docking involved in exocytosis / G protein-coupled dopamine receptor signaling pathway / cellular response to alkaloid / regulation of heart contraction / parallel fiber to Purkinje cell synapse / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / postsynaptic modulation of chemical synaptic transmission / positive regulation of vascular associated smooth muscle cell proliferation / G protein-coupled serotonin receptor binding / adenylate cyclase regulator activity / adenylate cyclase-inhibiting serotonin receptor signaling pathway / muscle contraction / locomotory behavior / negative regulation of insulin secretion / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / GABA-ergic synapse / cellular response to xenobiotic stimulus / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / cellular response to catecholamine stimulus / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / Inactivation, recovery and regulation of the phototransduction cascade / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / cell body / retina development in camera-type eye / G protein activity / GTPase binding / presynaptic membrane / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / chemical synaptic transmission / Ras protein signal transduction / postsynaptic membrane / Extra-nuclear estrogen signaling / cell population proliferation / G protein-coupled receptor signaling pathway / lysosomal membrane / GTPase activity / dendrite / synapse / GTP binding / protein-containing complex binding / glutamatergic synapse / endoplasmic reticulum / signal transduction Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.78 Å | |||||||||
 Authors Authors | Garcia-Nafria, J. / Nehme, R. / Edwards, P. / Tate, C.G. | |||||||||
| Funding support |  United Kingdom, 2items United Kingdom, 2items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2018 Journal: Nature / Year: 2018Title: Cryo-EM structure of the serotonin 5-HT receptor coupled to heterotrimeric G. Authors: Javier García-Nafría / Rony Nehmé / Patricia C Edwards / Christopher G Tate /  Abstract: G-protein-coupled receptors (GPCRs) form the largest family of receptors encoded by the human genome (around 800 genes). They transduce signals by coupling to a small number of heterotrimeric G ...G-protein-coupled receptors (GPCRs) form the largest family of receptors encoded by the human genome (around 800 genes). They transduce signals by coupling to a small number of heterotrimeric G proteins (16 genes encoding different α-subunits). Each human cell contains several GPCRs and G proteins. The structural determinants of coupling of G to four different GPCRs have been elucidated, but the molecular details of how the other G-protein classes couple to GPCRs are unknown. Here we present the cryo-electron microscopy structure of the serotonin 5-HT receptor (5-HTR) bound to the agonist donitriptan and coupled to an engineered G heterotrimer. In this complex, 5-HTR is in an active state; the intracellular domain of the receptor is in a similar conformation to that observed for the β-adrenoceptor (βAR) or the adenosine A receptor (AR) in complex with G. In contrast to the complexes with G, the gap between the receptor and the Gβ-subunit in the G-5-HTR complex precludes molecular contacts, and the interface between the Gα-subunit of G and the receptor is considerably smaller. These differences are likely to be caused by the differences in the interactions with the C terminus of the G α-subunit. The molecular variations between the interfaces of G and G in complex with GPCRs may contribute substantially to both the specificity of coupling and the kinetics of signalling. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6g79.cif.gz 6g79.cif.gz | 176.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6g79.ent.gz pdb6g79.ent.gz | 129.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6g79.json.gz 6g79.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/g7/6g79 https://data.pdbj.org/pub/pdb/validation_reports/g7/6g79 ftp://data.pdbj.org/pub/pdb/validation_reports/g7/6g79 ftp://data.pdbj.org/pub/pdb/validation_reports/g7/6g79 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4358MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10308 (Title: Cryo-EM structure of the serotonin 5-HT1B receptor coupled to heterotrimeric Go EMPIAR-10308 (Title: Cryo-EM structure of the serotonin 5-HT1B receptor coupled to heterotrimeric GoData size: 8.0 TB / Data #1: Unaligned movies [micrographs - multiframe] Data #2: Extracted particles [picked particles - multiframe - processed]) |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 37285.734 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNB1 / Production host: Homo sapiens (human) / Gene: GNB1 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: P62873 Trichoplusia ni (cabbage looper) / References: UniProt: P62873 |
|---|---|
| #2: Protein | Mass: 7845.078 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNG2 / Production host: Homo sapiens (human) / Gene: GNG2 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: P59768 Trichoplusia ni (cabbage looper) / References: UniProt: P59768 |
| #3: Protein | Mass: 25155.727 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNAO1 / Production host: Homo sapiens (human) / Gene: GNAO1 / Production host:  |
| #4: Protein | Mass: 41273.375 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: HTR1B, HTR1DB / Production host: Homo sapiens (human) / Gene: HTR1B, HTR1DB / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: P28222 Trichoplusia ni (cabbage looper) / References: UniProt: P28222 |
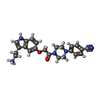
| #5: Chemical | ChemComp-EP5 / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight |
| ||||||||||||||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.5 | ||||||||||||||||||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||||||||||||||||||
| Specimen | Conc.: 2.2 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||||||||||||||
| Specimen support | Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277.15 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Cs: 2.7 mm / C2 aperture diameter: 50 µm |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 60 sec. / Electron dose: 30 e/Å2 / Detector mode: COUNTING / Film or detector model: FEI FALCON III (4k x 4k) / Num. of real images: 5737 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.78 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 730118 / Symmetry type: POINT |
 Movie
Movie Controller
Controller












 PDBj
PDBj