[English] 日本語
 Yorodumi
Yorodumi- PDB-6fug: Complement factor D in complex with the inhibitor 3-((3-((3-(amin... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6fug | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Complement factor D in complex with the inhibitor 3-((3-((3-(aminomethyl)phenyl)amino)-1H-pyrazolo[3,4-d]pyrimidin-4-yl)amino)phenol | |||||||||
 Components Components | Complement factor D | |||||||||
 Keywords Keywords | HYDROLASE / SERINE PROTEASE / inhibitor / complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationcomplement factor D / Alternative complement activation / complement activation, alternative pathway / complement activation / serine-type peptidase activity / platelet alpha granule lumen / protein maturation / response to bacterium / Platelet degranulation / secretory granule lumen ...complement factor D / Alternative complement activation / complement activation, alternative pathway / complement activation / serine-type peptidase activity / platelet alpha granule lumen / protein maturation / response to bacterium / Platelet degranulation / secretory granule lumen / ficolin-1-rich granule lumen / serine-type endopeptidase activity / Neutrophil degranulation / proteolysis / extracellular space / extracellular exosome / extracellular region Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.21 Å MOLECULAR REPLACEMENT / Resolution: 2.21 Å | |||||||||
 Authors Authors | Mac Sweeney, A. / Ostermann, N. / Vulpetti, A. / Maibaum, J. / Erbel, P. / Lorthiois, E. / Yoon, T. / Randl, S. / Ruedisser, S. | |||||||||
 Citation Citation |  Journal: ACS Med Chem Lett / Year: 2018 Journal: ACS Med Chem Lett / Year: 2018Title: Discovery and Design of First Benzylamine-Based Ligands Binding to an Unlocked Conformation of the Complement Factor D. Authors: Vulpetti, A. / Ostermann, N. / Randl, S. / Yoon, T. / Mac Sweeney, A. / Cumin, F. / Lorthiois, E. / Rudisser, S. / Erbel, P. / Maibaum, J. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6fug.cif.gz 6fug.cif.gz | 261 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6fug.ent.gz pdb6fug.ent.gz | 209.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6fug.json.gz 6fug.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fu/6fug https://data.pdbj.org/pub/pdb/validation_reports/fu/6fug ftp://data.pdbj.org/pub/pdb/validation_reports/fu/6fug ftp://data.pdbj.org/pub/pdb/validation_reports/fu/6fug | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6ftyC  6ftzC  6fuhC  6fuiC  6fujC  6futC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 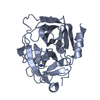
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | 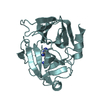
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: _ / Beg auth comp-ID: ILE / Beg label comp-ID: ILE / Refine code: _
|
 Movie
Movie Controller
Controller












 PDBj
PDBj