[English] 日本語
 Yorodumi
Yorodumi- PDB-6eg1: Crystal structure of Dpr2 Ig1-Ig2 in complex with DIP-Theta Ig1-Ig3 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6eg1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Dpr2 Ig1-Ig2 in complex with DIP-Theta Ig1-Ig3 | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | CELL ADHESION / Immunoglobulin Super-Family / Synaptic specification / nervous system development / cell-surface protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationDegradation of the extracellular matrix / Non-integrin membrane-ECM interactions / ECM proteoglycans / HS-GAG biosynthesis / HS-GAG degradation / Integrin cell surface interactions / neuron projection membrane / Glycosaminoglycan-protein linkage region biosynthesis / sensory perception of chemical stimulus / synapse organization ...Degradation of the extracellular matrix / Non-integrin membrane-ECM interactions / ECM proteoglycans / HS-GAG biosynthesis / HS-GAG degradation / Integrin cell surface interactions / neuron projection membrane / Glycosaminoglycan-protein linkage region biosynthesis / sensory perception of chemical stimulus / synapse organization / neuron projection / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.95 Å MOLECULAR REPLACEMENT / Resolution: 2.95 Å | |||||||||
 Authors Authors | Cosmanescu, F. / Patel, S. / Shapiro, L. | |||||||||
| Funding support |  United States, 1items United States, 1items
| |||||||||
 Citation Citation |  Journal: Neuron / Year: 2018 Journal: Neuron / Year: 2018Title: Neuron-Subtype-Specific Expression, Interaction Affinities, and Specificity Determinants of DIP/Dpr Cell Recognition Proteins. Authors: Cosmanescu, F. / Katsamba, P.S. / Sergeeva, A.P. / Ahlsen, G. / Patel, S.D. / Brewer, J.J. / Tan, L. / Xu, S. / Xiao, Q. / Nagarkar-Jaiswal, S. / Nern, A. / Bellen, H.J. / Zipursky, S.L. / ...Authors: Cosmanescu, F. / Katsamba, P.S. / Sergeeva, A.P. / Ahlsen, G. / Patel, S.D. / Brewer, J.J. / Tan, L. / Xu, S. / Xiao, Q. / Nagarkar-Jaiswal, S. / Nern, A. / Bellen, H.J. / Zipursky, S.L. / Honig, B. / Shapiro, L. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6eg1.cif.gz 6eg1.cif.gz | 232 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6eg1.ent.gz pdb6eg1.ent.gz | 185.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6eg1.json.gz 6eg1.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/eg/6eg1 https://data.pdbj.org/pub/pdb/validation_reports/eg/6eg1 ftp://data.pdbj.org/pub/pdb/validation_reports/eg/6eg1 ftp://data.pdbj.org/pub/pdb/validation_reports/eg/6eg1 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6efyC  6efzC  6eg0SC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Antibody / Protein , 2 types, 4 molecules ACBD
| #1: Antibody | Mass: 25771.260 Da / Num. of mol.: 2 / Fragment: UNP residues 103-323 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Gene: dpr2, BP1042, CG14067, CG14068, CT33638, Dmel\CG33507, Dpr-2, Dpr2, CG33507, Dmel_CG33507 Production host:  Homo sapiens (human) / References: UniProt: Q59DZ4 Homo sapiens (human) / References: UniProt: Q59DZ4#2: Protein | Mass: 34110.230 Da / Num. of mol.: 2 / Fragment: UNP residues 128-423 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Gene: DIP-theta, 31646, CG14008, CG14009, CG31646-RA, CT33566, Dmel\CG31646, CG31646, Dmel_CG31646 Production host:  Homo sapiens (human) / References: UniProt: Q9VMN6 Homo sapiens (human) / References: UniProt: Q9VMN6 |
|---|
-Sugars , 6 types, 12 molecules 
| #3: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta- ...2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose #4: Polysaccharide | #5: Polysaccharide | #6: Polysaccharide | alpha-L-fucopyranose-(1-6)-2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #7: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | #11: Sugar | ChemComp-NAG / | |
|---|
-Non-polymers , 4 types, 60 molecules 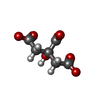

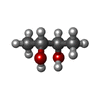




| #8: Chemical | | #9: Chemical | #10: Chemical | #12: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.28 Å3/Da / Density % sol: 71.27 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop Details: 18% PEG3350, 0.2 M triammonium citrate, pH 6.5, cryoprotectant: 15% (2R,3R)-(-)-2,3-butanediol |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 24-ID-C / Wavelength: 0.9792 Å / Beamline: 24-ID-C / Wavelength: 0.9792 Å |
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Mar 17, 2017 |
| Radiation | Monochromator: Cryo-cooled double crystal Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9792 Å / Relative weight: 1 |
| Reflection | Resolution: 2.95→120 Å / Num. obs: 43769 / % possible obs: 99.8 % / Redundancy: 5.2 % / Rmerge(I) obs: 0.121 / Net I/σ(I): 11.9 |
| Reflection shell | Resolution: 2.95→3.06 Å / Rmerge(I) obs: 0.715 / Num. unique obs: 4518 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 6EG0 Resolution: 2.95→19.959 Å / SU ML: 0.33 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 22.64
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.95→19.959 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj














