+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6cnq | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
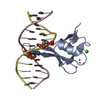
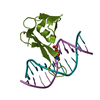
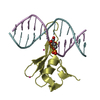
| Title | MBD2 in complex with methylated DNA | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | TRANSCRIPTION/DNA / dna methylation / dna binding / Structural Genomics / Structural Genomics Consortium / SGC / TRANSCRIPTION-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsatellite DNA binding / ventricular cardiac muscle tissue development / NuRD complex / siRNA binding / maternal behavior / C2H2 zinc finger domain binding / methyl-CpG binding / DNA methylation-dependent constitutive heterochromatin formation / embryonic organ development / response to mechanical stimulus ...satellite DNA binding / ventricular cardiac muscle tissue development / NuRD complex / siRNA binding / maternal behavior / C2H2 zinc finger domain binding / methyl-CpG binding / DNA methylation-dependent constitutive heterochromatin formation / embryonic organ development / response to mechanical stimulus / positive regulation of Wnt signaling pathway / heterochromatin / RNA Polymerase I Promoter Opening / response to nutrient levels / NoRC negatively regulates rRNA expression / Wnt signaling pathway / response to estradiol / regulation of cell population proliferation / protein-containing complex assembly / molecular adaptor activity / chromatin remodeling / protein domain specific binding / negative regulation of DNA-templated transcription / mRNA binding / chromatin binding / positive regulation of DNA-templated transcription / chromatin / negative regulation of transcription by RNA polymerase II / protein-containing complex / nucleoplasm / identical protein binding / nucleus / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human)synthetic construct (others) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.151 Å MOLECULAR REPLACEMENT / Resolution: 2.151 Å | |||||||||
 Authors Authors | Liu, K. / Xu, C. / Min, J. / Structural Genomics Consortium (SGC) | |||||||||
 Citation Citation |  Journal: J. Biol. Chem. / Year: 2018 Journal: J. Biol. Chem. / Year: 2018Title: Structural basis for the ability of MBD domains to bind methyl-CG and TG sites in DNA. Authors: Liu, K. / Xu, C. / Lei, M. / Yang, A. / Loppnau, P. / Hughes, T.R. / Min, J. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6cnq.cif.gz 6cnq.cif.gz | 123.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6cnq.ent.gz pdb6cnq.ent.gz | 92.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6cnq.json.gz 6cnq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/cn/6cnq https://data.pdbj.org/pub/pdb/validation_reports/cn/6cnq ftp://data.pdbj.org/pub/pdb/validation_reports/cn/6cnq ftp://data.pdbj.org/pub/pdb/validation_reports/cn/6cnq | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6c1aC  6c1tC  6c1uC  6c1vC  6cnpC  6c2k S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 8791.171 Da / Num. of mol.: 2 / Fragment: residues 143-220 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: MBD2 / Plasmid: pET28-MHL / Production host: Homo sapiens (human) / Gene: MBD2 / Plasmid: pET28-MHL / Production host:  #2: DNA chain | Mass: 3677.419 Da / Num. of mol.: 4 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) #3: Chemical | ChemComp-UNX / #4: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.2 Å3/Da / Density % sol: 61.6 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion / Details: 20% PEG-3350, 0.2M ammonium formate |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 19-ID / Wavelength: 0.97918 Å / Beamline: 19-ID / Wavelength: 0.97918 Å | ||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Jun 19, 2013 | ||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Scattering type: x-ray | ||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.97918 Å / Relative weight: 1 | ||||||||||||||||||||||||
| Reflection | Resolution: 2.15→40.58 Å / Num. obs: 20266 / % possible obs: 99.9 % / Redundancy: 5.6 % / Biso Wilson estimate: 52.8 Å2 / CC1/2: 0.999 / Rmerge(I) obs: 0.049 / Rpim(I) all: 0.023 / Rrim(I) all: 0.055 / Net I/σ(I): 18.1 / Num. measured all: 114295 / Scaling rejects: 0 | ||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: earlier version of PDB entry 6C2K, unpublished DNA model  6c2k Resolution: 2.151→24.344 Å / SU ML: 0.33 / Cross valid method: FREE R-VALUE / σ(F): 1.99 / Phase error: 29.05 Details: arp/warp was used in map improvement mode. refmac was used at intermediate stages of refinement. coot was used for interactive model building. Model geometry was assessed on the molprobity server.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 123.47 Å2 / Biso mean: 63.028 Å2 / Biso min: 35.57 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.151→24.344 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 10
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj









































