[English] 日本語
 Yorodumi
Yorodumi- PDB-6ayl: Human adipocyte lipid-binding protein FABP4 in complex with fluor... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6ayl | ||||||
|---|---|---|---|---|---|---|---|
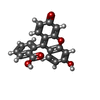
| Title | Human adipocyte lipid-binding protein FABP4 in complex with fluorescein | ||||||
 Components Components | Fatty acid-binding protein, adipocyte | ||||||
 Keywords Keywords | LIPID BINDING PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology informationhormone receptor binding / long-chain fatty acid transmembrane transporter activity / long-chain fatty acid binding / cellular response to lithium ion / Triglyceride catabolism / white fat cell differentiation / long-chain fatty acid transport / fatty acid transport / brown fat cell differentiation / lipid droplet ...hormone receptor binding / long-chain fatty acid transmembrane transporter activity / long-chain fatty acid binding / cellular response to lithium ion / Triglyceride catabolism / white fat cell differentiation / long-chain fatty acid transport / fatty acid transport / brown fat cell differentiation / lipid droplet / cholesterol homeostasis / fatty acid binding / response to bacterium / Transcriptional regulation of white adipocyte differentiation / positive regulation of inflammatory response / cellular response to tumor necrosis factor / positive regulation of cold-induced thermogenesis / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / negative regulation of DNA-templated transcription / extracellular exosome / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.86 Å MOLECULAR REPLACEMENT / Resolution: 1.86 Å | ||||||
 Authors Authors | Pozharski, E. | ||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: To be published Authors: Pozharski, E. / St John, F.S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6ayl.cif.gz 6ayl.cif.gz | 43.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6ayl.ent.gz pdb6ayl.ent.gz | 27.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6ayl.json.gz 6ayl.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6ayl_validation.pdf.gz 6ayl_validation.pdf.gz | 1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6ayl_full_validation.pdf.gz 6ayl_full_validation.pdf.gz | 1 MB | Display | |
| Data in XML |  6ayl_validation.xml.gz 6ayl_validation.xml.gz | 8 KB | Display | |
| Data in CIF |  6ayl_validation.cif.gz 6ayl_validation.cif.gz | 10.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ay/6ayl https://data.pdbj.org/pub/pdb/validation_reports/ay/6ayl ftp://data.pdbj.org/pub/pdb/validation_reports/ay/6ayl ftp://data.pdbj.org/pub/pdb/validation_reports/ay/6ayl | HTTPS FTP |
-Related structure data
| Related structure data |  3rzyS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 15485.729 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: FABP4 / Production host: Homo sapiens (human) / Gene: FABP4 / Production host:  | ||
|---|---|---|---|
| #2: Chemical | | #3: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.13 Å3/Da / Density % sol: 42.25 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 6.5 / Details: 1.6 M sodium citrate, pH 6.5 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRL SSRL  / Beamline: BL7-1 / Wavelength: 1.1271 Å / Beamline: BL7-1 / Wavelength: 1.1271 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Jun 16, 2012 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.1271 Å / Relative weight: 1 |
| Reflection | Resolution: 1.86→75 Å / Num. obs: 11207 / % possible obs: 96.3 % / Redundancy: 6.3 % / Biso Wilson estimate: 32.84 Å2 / CC1/2: 0.999 / Rmerge(I) obs: 0.068 / Rpim(I) all: 0.042 / Net I/av σ(I): 9.7 / Net I/σ(I): 13.9 |
| Reflection shell | Resolution: 1.86→1.9 Å / Redundancy: 4.4 % / Rmerge(I) obs: 1.204 / Mean I/σ(I) obs: 0.9 / Num. unique obs: 501 / CC1/2: 0.391 / Rpim(I) all: 0.921 / % possible all: 73.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3RZY Resolution: 1.86→37.49 Å / Cor.coef. Fo:Fc: 0.923 / Cor.coef. Fo:Fc free: 0.924 / Rfactor Rfree error: 0 / SU R Cruickshank DPI: 0.162 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.169 / SU Rfree Blow DPI: 0.141 / SU Rfree Cruickshank DPI: 0.138
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 42.8 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.32 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 1.86→37.49 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.86→2.04 Å / Rfactor Rfree error: 0 / Total num. of bins used: 6
|
 Movie
Movie Controller
Controller










 PDBj
PDBj